Contraction regulates site-specific phosphorylation of TBC1D1 in skeletal muscle
- PMID: 20701589
- PMCID: PMC2947193
- DOI: 10.1042/BJ20101100
Contraction regulates site-specific phosphorylation of TBC1D1 in skeletal muscle
Abstract
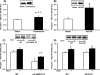
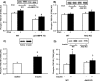
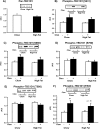
TBC1D1 (tre-2/USP6, BUB2, cdc16 domain family member 1) is a Rab-GAP (GTPase-activating protein) that is highly expressed in skeletal muscle, but little is known about TBC1D1 regulation and function. We studied TBC1D1 phosphorylation on three predicted AMPK (AMP-activated protein kinase) phosphorylation sites (Ser231, Ser660 and Ser700) and one predicted Akt phosphorylation site (Thr590) in control mice, AMPKα2 inactive transgenic mice (AMPKα2i TG) and Akt2-knockout mice (Akt2 KO). Muscle contraction significantly increased TBC1D1 phosphorylation on Ser231 and Ser660, tended to increase Ser700 phosphorylation, but had no effect on Thr590. AICAR (5-aminoimidazole-4-carboxyamide ribonucleoside) also increased phosphorylation on Ser231, Ser660 and Ser700, but not Thr590, whereas insulin only increased Thr590 phosphorylation. Basal and contraction-stimulated TBC1D1 Ser231, Ser660 and Ser700 phosphorylation were greatly reduced in AMPKα2i TG mice, although contraction still elicited a small increase in phosphorylation. Akt2 KO mice had blunted insulin-stimulated TBC1D1 Thr590 phosphorylation. Contraction-stimulated TBC1D1 Ser231 and Ser660 phosphorylation were normal in high-fat-fed mice. Glucose uptake in vivo was significantly decreased in tibialis anterior muscles overexpressing TBC1D1 mutated on four predicted AMPK phosphorylation sites. In conclusion, contraction causes site-specific phosphorylation of TBC1D1 in skeletal muscle, and TBC1D1 phosphorylation on AMPK sites regulates contraction-stimulated glucose uptake. AMPK and Akt regulate TBC1D1 phosphorylation, but there must be additional upstream kinases that mediate TBC1D1 phosphorylation in skeletal muscle.
Figures


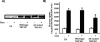
References
-
- DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. P. The effect of insulin on the disposal of intravenous glucoseResults from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. - PubMed
-
- Baron A. D., Brechtel G., Wallace P., Edelman S. V. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am. J. Physiol. 1988;255:E769–E774. - PubMed
-
- DeFronzo R. A., Simonson D., Ferrannini E. Hepatic and peripheral insulin resistance: a common feature of type II (non-insulin-dependent) and type I (insulin dependent) diabetes mellitus. Diabetologia. 1982;23:313–319. - PubMed
-
- Dohm G. L., Tapscott E. B., Pories W. J., Dabbs D. J., Flickinger E. G., Meelheim D., Fushiki T., Atkinson S. M., Elton C. W., Caro J. F. An in vitro human muscle preparation suitable for metabolic studies. Decreased insulin stimulation of glucose transport in muscle from morbidly obese and diabetic subjects. J. Clin. Invest. 1988;82:486–494. - PMC - PubMed
-
- Kennedy J. W., Hirshman M. F., Gervino E. V., Ocel J. V., Forse R. A., Hoenig S. J., Aronson D., Goodyear L. J., Horton E. S. Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes. 1999;48:1192–1197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

