Fat tissue, aging, and cellular senescence
- PMID: 20701600
- PMCID: PMC2941545
- DOI: 10.1111/j.1474-9726.2010.00608.x
Fat tissue, aging, and cellular senescence
Abstract
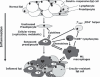
Fat tissue, frequently the largest organ in humans, is at the nexus of mechanisms involved in longevity and age-related metabolic dysfunction. Fat distribution and function change dramatically throughout life. Obesity is associated with accelerated onset of diseases common in old age, while fat ablation and certain mutations affecting fat increase life span. Fat cells turn over throughout the life span. Fat cell progenitors, preadipocytes, are abundant, closely related to macrophages, and dysdifferentiate in old age, switching into a pro-inflammatory, tissue-remodeling, senescent-like state. Other mesenchymal progenitors also can acquire a pro-inflammatory, adipocyte-like phenotype with aging. We propose a hypothetical model in which cellular stress and preadipocyte overutilization with aging induce cellular senescence, leading to impaired adipogenesis, failure to sequester lipotoxic fatty acids, inflammatory cytokine and chemokine generation, and innate and adaptive immune response activation. These pro-inflammatory processes may amplify each other and have systemic consequences. This model is consistent with recent concepts about cellular senescence as a stress-responsive, adaptive phenotype that develops through multiple stages, including major metabolic and secretory readjustments, which can spread from cell to cell and can occur at any point during life. Senescence could be an alternative cell fate that develops in response to injury or metabolic dysfunction and might occur in nondividing as well as dividing cells. Consistent with this, a senescent-like state can develop in preadipocytes and fat cells from young obese individuals. Senescent, pro-inflammatory cells in fat could have profound clinical consequences because of the large size of the fat organ and its central metabolic role.
© 2010 The Authors Aging Cell © 2010 Blackwell Publishing Ltd/Anatomical Society of Great Britain and Ireland.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

