Karyopherin alpha7 (KPNA7), a divergent member of the importin alpha family of nuclear import receptors
- PMID: 20701745
- PMCID: PMC2929220
- DOI: 10.1186/1471-2121-11-63
Karyopherin alpha7 (KPNA7), a divergent member of the importin alpha family of nuclear import receptors
Abstract
Background: Classical nuclear localization signal (NLS) dependent nuclear import is carried out by a heterodimer of importin alpha and importin beta. NLS cargo is recognized by importin alpha, which is bound by importin beta. Importin beta mediates translocation of the complex through the central channel of the nuclear pore, and upon reaching the nucleus, RanGTP binding to importin beta triggers disassembly of the complex. To date, six importin alpha family members, encoded by separate genes, have been described in humans.
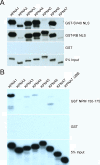
Results: We sequenced and characterized a seventh member of the importin alpha family of transport factors, karyopherin alpha 7 (KPNA7), which is most closely related to KPNA2. The domain of KPNA7 that binds Importin beta (IBB) is divergent, and shows stronger binding to importin beta than the IBB domains from of other importin alpha family members. With regard to NLS recognition, KPNA7 binds to the retinoblastoma (RB) NLS to a similar degree as KPNA2, but it fails to bind the SV40-NLS and the human nucleoplasmin (NPM) NLS. KPNA7 shows a predominantly nuclear distribution under steady state conditions, which contrasts with KPNA2 which is primarily cytoplasmic.
Conclusion: KPNA7 is a novel importin alpha family member in humans that belongs to the importin alpha2 subfamily. KPNA7 shows different subcellular localization and NLS binding characteristics compared to other members of the importin alpha family. These properties suggest that KPNA7 could be specialized for interactions with select NLS-containing proteins, potentially impacting developmental regulation.
Figures





References
-
- Harel A, Forbes DJ. Importin beta: conducting a much larger cellular symphony. Mol Cell. 2004;16(3):319–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

