Simulation methods with extended stability for stiff biochemical Kinetics
- PMID: 20701766
- PMCID: PMC3225827
- DOI: 10.1186/1752-0509-4-110
Simulation methods with extended stability for stiff biochemical Kinetics
Abstract
Background: With increasing computer power, simulating the dynamics of complex systems in chemistry and biology is becoming increasingly routine. The modelling of individual reactions in (bio)chemical systems involves a large number of random events that can be simulated by the stochastic simulation algorithm (SSA). The key quantity is the step size, or waiting time, tau, whose value inversely depends on the size of the propensities of the different channel reactions and which needs to be re-evaluated after every firing event. Such a discrete event simulation may be extremely expensive, in particular for stiff systems where tau can be very short due to the fast kinetics of some of the channel reactions. Several alternative methods have been put forward to increase the integration step size. The so-called tau-leap approach takes a larger step size by allowing all the reactions to fire, from a Poisson or Binomial distribution, within that step. Although the expected value for the different species in the reactive system is maintained with respect to more precise methods, the variance at steady state can suffer from large errors as tau grows.
Results: In this paper we extend Poisson tau-leap methods to a general class of Runge-Kutta (RK) tau-leap methods. We show that with the proper selection of the coefficients, the variance of the extended tau-leap can be well-behaved, leading to significantly larger step sizes.
Conclusions: The benefit of adapting the extended method to the use of RK frameworks is clear in terms of speed of calculation, as the number of evaluations of the Poisson distribution is still one set per time step, as in the original tau-leap method. The approach paves the way to explore new multiscale methods to simulate (bio)chemical systems.
Figures

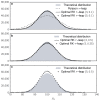
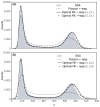
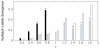
Similar articles
-
Unbiased tau-leap methods for stochastic simulation of chemically reacting systems.J Chem Phys. 2008 Apr 21;128(15):154112. doi: 10.1063/1.2894479. J Chem Phys. 2008. PMID: 18433195
-
Generalized binomial tau-leap method for biochemical kinetics incorporating both delay and intrinsic noise.J Chem Phys. 2008 May 28;128(20):205107. doi: 10.1063/1.2919124. J Chem Phys. 2008. PMID: 18513050
-
Efficient binomial leap method for simulating chemical kinetics.J Chem Phys. 2007 Jun 14;126(22):224109. doi: 10.1063/1.2741252. J Chem Phys. 2007. PMID: 17581046
-
Stochastic simulation of chemical kinetics.Annu Rev Phys Chem. 2007;58:35-55. doi: 10.1146/annurev.physchem.58.032806.104637. Annu Rev Phys Chem. 2007. PMID: 17037977 Review.
-
Simulation Strategies for Calcium Microdomains and Calcium Noise.Adv Exp Med Biol. 2020;1131:771-797. doi: 10.1007/978-3-030-12457-1_31. Adv Exp Med Biol. 2020. PMID: 31646534 Review.
Cited by
-
A higher-order numerical framework for stochastic simulation of chemical reaction systems.BMC Syst Biol. 2012 Jul 15;6:85. doi: 10.1186/1752-0509-6-85. BMC Syst Biol. 2012. PMID: 23256696 Free PMC article.
-
Stochastic simulation in systems biology.Comput Struct Biotechnol J. 2014 Oct 30;12(20-21):14-25. doi: 10.1016/j.csbj.2014.10.003. eCollection 2014 Nov. Comput Struct Biotechnol J. 2014. PMID: 25505503 Free PMC article. Review.
-
Efficient simulation of stochastic chemical kinetics with the Stochastic Bulirsch-Stoer extrapolation method.BMC Syst Biol. 2014 Jun 18;8:71. doi: 10.1186/1752-0509-8-71. BMC Syst Biol. 2014. PMID: 24939084 Free PMC article.
References
-
- Turner TE, Schnell S, Burrage K. Stochastic approaches for modelling in vivo reactions. Computational Biology and Chemistry. 2004;28(3):165–178. doi: 10.1016/j.compbiolchem.2004.05.001. http://www.sciencedirect.com/science/article/B73G2-4CS4GV4-1/2/f17f5571a... - DOI - PubMed
-
- Klipp E, Herwig R, Kowald A, Wierling C, Lehrach H. Systems biology in practice. Wiley-VCH Weinheim; 2005. full_text.
-
- Wilkinson D. Stochastic Modelling for Systems Biology. CRC Press; 2006.
-
- Villà J, Warshel A. Energetics and Dynamics of Enzymatic Reactions. J Phys Chem B. 2001;105:7887–907. doi: 10.1021/jp011048h. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

