The use of exenatide in severely burned pediatric patients
- PMID: 20701787
- PMCID: PMC2945137
- DOI: 10.1186/cc9222
The use of exenatide in severely burned pediatric patients
Abstract
Introduction: Intensive insulin treatment (IIT) has been shown to improve outcomes post-burn in severely burnt patients. However, it increases the incidence of hypoglycemia and is associated with risks and complications. We hypothesized that exenatide would decrease plasma glucose levels post-burn to levels similar to those achieved with IIT, and reduce the amount of exogenous insulin administered.
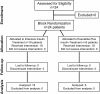
Methods: This open-label study included 24 severely burned pediatric patients. Six were randomized to receive exenatide, and 18 received IIT during acute hospitalization (block randomization). Exenatide and insulin were administered to maintain glucose levels between 80 and 140 mg/dl. We determined 6 AM, daily average, maximum and minimum glucose levels. Variability was determined using mean amplitude of glucose excursions (MAGE) and percentage of coefficient of variability. The amount of administered insulin was compared in both groups.
Results: Glucose values and variability were similar in both groups: Daily average was 130 ± 28 mg/dl in the intervention group and 138 ± 25 mg/dl in the control group (P = 0.31), MAGE 41 ± 6 vs. 45 ± 12 (respectively). However, administered insulin was significantly lower in the exenatide group than in the IIT group: 22 ± 14 IU patients/day in the intervention group and 76 ± 11 IU patients/day in the control group (P = 0.01). The incidence rate of hypoglycemia was similar in both groups (0.38 events/patient-month).
Conclusions: Patients receiving exenatide received significantly lower amounts of exogenous insulin to control plasma glucose levels. Exenatide was well tolerated and potentially represents a novel agent to attenuate hyperglycemia in the critical care setting.
Trial registration: NCT00673309.
Figures






Comment in
-
The therapeutic potential of a venomous lizard: the use of glucagon-like peptide-1 analogues in the critically ill.Crit Care. 2010;14(5):1004. doi: 10.1186/cc9281. Epub 2010 Oct 21. Crit Care. 2010. PMID: 20979668 Free PMC article.
Similar articles
-
Management of hyperglycemia with the administration of intravenous exenatide to patients in the cardiac intensive care unit.Endocr Pract. 2013 Jan-Feb;19(1):81-90. doi: 10.4158/EP12196.OR. Endocr Pract. 2013. PMID: 23186969
-
Comparison of exenatide with biphasic insulin aspart 30 on glucose variability in type 2 diabetes: study protocol for a randomized controlled trial.Trials. 2016 Mar 24;17:160. doi: 10.1186/s13063-016-1258-8. Trials. 2016. PMID: 27009108 Free PMC article. Clinical Trial.
-
Efficacy and safety of biphasic insulin aspart 70/30 versus exenatide in subjects with type 2 diabetes failing to achieve glycemic control with metformin and a sulfonylurea.Curr Med Res Opin. 2009 Jan;25(1):65-75. doi: 10.1185/03007990802597951. Curr Med Res Opin. 2009. PMID: 19210140 Clinical Trial.
-
Rationale and design of Short-Term EXenatide therapy in Acute ischaemic Stroke (STEXAS): a randomised, open-label, parallel-group study.BMJ Open. 2016 Feb 24;6(2):e008203. doi: 10.1136/bmjopen-2015-008203. BMJ Open. 2016. PMID: 26911582 Free PMC article. Clinical Trial.
-
[Exenatide--an alternative to insulin in the treatment of type 2 diabetes?].Ugeskr Laeger. 2008 Sep 22;170(39):3039-43. Ugeskr Laeger. 2008. PMID: 18822227 Review. Danish.
Cited by
-
The therapeutic potential of a venomous lizard: the use of glucagon-like peptide-1 analogues in the critically ill.Crit Care. 2010;14(5):1004. doi: 10.1186/cc9281. Epub 2010 Oct 21. Crit Care. 2010. PMID: 20979668 Free PMC article.
-
Is incretin-based therapy ready for the care of hospitalized patients with type 2 diabetes?: The time has come for GLP-1 receptor agonists!Diabetes Care. 2013 Jul;36(7):2107-11. doi: 10.2337/dc12-2060. Diabetes Care. 2013. PMID: 23801800 Free PMC article.
-
Current problems in burn hypermetabolism.Curr Probl Surg. 2020 Jan;57(1):100709. doi: 10.1016/j.cpsurg.2019.100709. Epub 2019 Nov 11. Curr Probl Surg. 2020. PMID: 32033707 Free PMC article. Review. No abstract available.
-
Exendin-4 Exacerbates Burn-Induced Morbidity in Mice by Activation of the Sympathetic Nervous System.Mediators Inflamm. 2019 Jan 17;2019:2750528. doi: 10.1155/2019/2750528. eCollection 2019. Mediators Inflamm. 2019. PMID: 30800001 Free PMC article.
-
The metabolic stress response to burn trauma: current understanding and therapies.Lancet. 2016 Oct 1;388(10052):1417-1426. doi: 10.1016/S0140-6736(16)31469-6. Lancet. 2016. PMID: 27707498 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

