Mechanistic interplay among the M184I HIV-1 reverse transcriptase mutant, the central polypurine tract, cellular dNTP concentrations and drug sensitivity
- PMID: 20701944
- PMCID: PMC3097044
- DOI: 10.1016/j.virol.2010.07.028
Mechanistic interplay among the M184I HIV-1 reverse transcriptase mutant, the central polypurine tract, cellular dNTP concentrations and drug sensitivity
Abstract
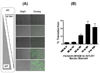
We recently reported that the M184I 3TC resistant mutation reduces RT binding affinity to dNTP substrates. First, the HIV-1 M184I mutant vector displays reduced transduction efficiency compared to wild type (WT) RT vector, which could be rescued by both elevating the cellular dNTP concentration and incorporating WT RT molecules into the M184I vector particles. Second, the central polypurine tract (cPPT) mutation and M184I mutation additively reduced the vector transduction to almost undetectable levels, particularly in nondividing cells. Third, the M184I (-) cPPT vector became significantly more sensitive to 3TC than the M184I (+) cPPT vector, but not to AZT or Nevirapine in the dividing cells. Finally, this 3TC sensitizing effect of the cPPT inactivation of the M184I vector was reversed by elevating the dCTP level, but not by the other three dNTPs. These data support a mechanistic interaction between cPPT and M184I RT with respect to viral replication and sensitivity to 3TC.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures




References
-
- Aquaro S, Svicher V, Ceccherini-Silberstein F, Cenci A, Marcuccilli F, Giannella S, Marcon L, Caliò R, Balzarini J, Perno CF. Limited development and progression of resistance of HIV-1 to the nucleoside analogue reverse transcriptase inhibitor lamivudine in human primary macrophages. J Antimicrob Chemother. 2005;55(6):872–878. - PubMed
-
- Crowe S, Zhu T, Muller WA. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J Leukoc Biol. 2003;74(5):635–641. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

