Role of H-1 and H-2 subunits of soybean seed ferritin in oxidative deposition of iron in protein
- PMID: 20702403
- PMCID: PMC2952209
- DOI: 10.1074/jbc.M110.130435
Role of H-1 and H-2 subunits of soybean seed ferritin in oxidative deposition of iron in protein
Abstract
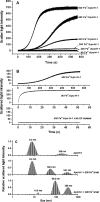
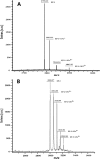
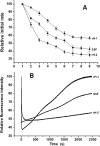
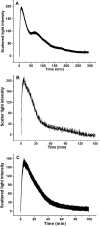
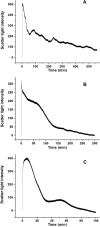
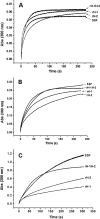
Naturally occurring phytoferritin is a heteropolymer consisting of two different H-type subunits, H-1 and H-2. Prior to this study, however, the function of the two subunits in oxidative deposition of iron in ferritin was unknown. The data show that, upon aerobic addition of 48-200 Fe(2+)/shell to apoferritin, iron oxidation occurs only at the diiron ferroxidase center of recombinant H1 (rH-1). In addition to the diiron ferroxidase mechanism, such oxidation is catalyzed by the extension peptide (a specific domain found in phytoferritin) of rH-2, because the H-1 subunit is able to remove Fe(3+) from the center to the inner cavity better than the H-2 subunit. These findings support the idea that the H-1 and H-2 subunits play different roles in iron mineralization in protein. Interestingly, at medium iron loading (200 irons/shell), wild-type (WT) soybean seed ferritin (SSF) exhibits a stronger activity in catalyzing iron oxidation (1.10 ± 0.13 μm iron/subunit/s) than rH-1 (0.59 ± 0.07 μm iron/subunit/s) and rH-2 (0.48 ± 0.04 μm iron/subunit/s), demonstrating that a synergistic interaction exists between the H-1 and H-2 subunits in SSF during iron mineralization. Such synergistic interaction becomes considerably stronger at high iron loading (400 irons/shell) as indicated by the observation that the iron oxidation activity of WT SSF is ∼10 times larger than those of rH-1 and rH-2. This helps elucidate the widespread occurrence of heteropolymeric ferritins in plants.
Figures


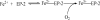
Similar articles
-
A novel strategy of natural plant ferritin to protect DNA from oxidative damage during iron oxidation.Free Radic Biol Med. 2012 Jul 15;53(2):375-82. doi: 10.1016/j.freeradbiomed.2012.05.003. Epub 2012 May 8. Free Radic Biol Med. 2012. PMID: 22580341
-
Stability and iron oxidation properties of a novel homopolymeric plant ferritin from adzuki bean seeds: a comparative analysis with recombinant soybean seed H-1 chain ferritin.Biochim Biophys Acta. 2013 Apr;1830(4):2946-53. doi: 10.1016/j.bbagen.2013.01.004. Epub 2013 Jan 11. Biochim Biophys Acta. 2013. PMID: 23313843
-
Two different H-type subunits from pea seed (Pisum sativum) ferritin that are responsible for fast Fe(II) oxidation.Biochimie. 2009 Feb;91(2):230-9. doi: 10.1016/j.biochi.2008.09.008. Epub 2008 Oct 17. Biochimie. 2009. PMID: 18984027
-
The iron redox and hydrolysis chemistry of the ferritins.Biochim Biophys Acta. 2010 Aug;1800(8):719-31. doi: 10.1016/j.bbagen.2010.03.021. Epub 2010 Apr 9. Biochim Biophys Acta. 2010. PMID: 20382203 Review.
-
Structure, function, and nutrition of phytoferritin: a newly functional factor for iron supplement.Crit Rev Food Sci Nutr. 2014;54(10):1342-52. doi: 10.1080/10408398.2011.635914. Crit Rev Food Sci Nutr. 2014. PMID: 24564591 Review.
Cited by
-
Interactions of β-Conglycinin (7S) with Different Phenolic Acids-Impact on Structural Characteristics and Proteolytic Degradation of Proteins.Int J Mol Sci. 2016 Oct 2;17(10):1671. doi: 10.3390/ijms17101671. Int J Mol Sci. 2016. PMID: 27706090 Free PMC article.
-
Ferritin protein nanocages use ion channels, catalytic sites, and nucleation channels to manage iron/oxygen chemistry.Curr Opin Chem Biol. 2011 Apr;15(2):304-11. doi: 10.1016/j.cbpa.2011.01.004. Epub 2011 Feb 4. Curr Opin Chem Biol. 2011. PMID: 21296609 Free PMC article. Review.
-
Thermostability of protein nanocages: the effect of natural extra peptide on the exterior surface.RSC Adv. 2019 Aug 9;9(43):24777-24782. doi: 10.1039/c9ra04785a. eCollection 2019 Aug 8. RSC Adv. 2019. PMID: 35528680 Free PMC article.
-
The extension peptide of plant ferritin from sea lettuce contributes to shell stability and surface hydrophobicity.Protein Sci. 2012 Jun;21(6):786-96. doi: 10.1002/pro.2061. Epub 2012 Apr 18. Protein Sci. 2012. PMID: 22419613 Free PMC article.
-
Pea Ferritin Stability under Gastric pH Conditions Determines the Mechanism of Iron Uptake in Caco-2 Cells.J Nutr. 2018 Aug 1;148(8):1229-1235. doi: 10.1093/jn/nxy096. J Nutr. 2018. PMID: 29939292 Free PMC article.
References
-
- Harrison P. M., Arosio P. (1996) Biochim. Biophys. Acta 1275, 161–203 - PubMed
-
- Le Brun N. E., Crow A., Murphy M. E., Mauk A. G., Moore G. R. (2010) Biochim. Biophys. Acta 1800, 732–744 - PubMed
-
- Zhao G., Ceci P., Ilari A., Giangiacomo L., Laue T. M., Chiancone E., Chasteen N. D. (2002) J. Biol. Chem. 277, 27689–27696 - PubMed
-
- Solomon E. I., Brunold T. C., Davis M. I., Kemsley J. N., Lee S. K., Lehnert N., Neese F., Skulan A. J., Yang Y. S., Zhou J. (2000) Chem. Rev. 100, 235–350 - PubMed
-
- Kopp D. A., Lippard S. J. (2002) Curr. Opin. Chem. Biol. 6, 568–576 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

