CCAAT/enhancer-binding protein β and NF-κB mediate high level expression of chemokine genes CCL3 and CCL4 by human chondrocytes in response to IL-1β
- PMID: 20702408
- PMCID: PMC2963416
- DOI: 10.1074/jbc.M110.130377
CCAAT/enhancer-binding protein β and NF-κB mediate high level expression of chemokine genes CCL3 and CCL4 by human chondrocytes in response to IL-1β
Abstract
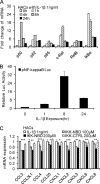
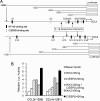
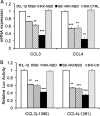
A large set of chemokines is highly up-regulated in human chondrocytes in response to IL-1β (Sandell, L. J., Xing, X., Franz, C., Davies, S., Chang, L. W., and Patra, D. (2008) Osteoarthr. Cartil. 16, 1560-1571). To investigate the mechanism of transcriptional regulation, deletion constructs of selected chemokine gene promoters, the human CCL3 (MIP-1α) and CCL4 (MIP-1β), were transfected into human chondrocytes with or without IL-1β. The results show that an IL-1β-responsive element is located between bp -300 and -140 of the CCL3 promoter and between bp -222 and -100 of the CCL4 promoter. Because both of these elements contain CCAAT/enhancer-binding protein β (C/EBPβ) motifs, the function of C/EBPβ was examined. IL-1β stimulated the expression of C/EBPβ, and the direct binding of C/EBPβ to the C/EBPβ motif was confirmed by EMSA and ChIP analyses. The -300 bp CCL3 promoter and -222 bp CCL4 promoter were strongly up-regulated by co-transfection with the C/EBPβ expression vector. Mutation of the C/EBPβ motif and reduction of C/EBPβ expression by siRNA decreased the up-regulation. Additionally, another cytokine-related transcription factor, NF-κB, was also shown to be involved in the up-regulation of chemokines in response to IL-1β, and the binding site was identified. The regulation of C/EBPβ and NF-κB was confirmed by the inhibition by C/EBPβ and NF-κB and by transfection with C/EBPβ and NF-κB expression vectors in the presence or absence of IL-1β. Taken together, our results suggest that C/EBPβ and NF-κB are both involved in the IL-1β-responsive up-regulation of chemokine genes in human chondrocytes. Time course experiments indicated that C/EBPβ gradually and steadily induces chemokine up-regulation, whereas NF-κB activity was highest at the early stage of chemokine up-regulation.
Figures





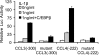



Similar articles
-
Resistin stimulates expression of chemokine genes in chondrocytes via combinatorial regulation of C/EBPβ and NF-κB.Int J Mol Sci. 2014 Sep 26;15(10):17242-55. doi: 10.3390/ijms151017242. Int J Mol Sci. 2014. PMID: 25264740 Free PMC article.
-
Tumor necrosis factor α- and interleukin-1β-dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1.Arthritis Rheum. 2013 Mar;65(3):832-42. doi: 10.1002/art.37819. Arthritis Rheum. 2013. PMID: 23233369 Free PMC article.
-
SOCS1 suppresses IL-1β-induced C/EBPβ expression via transcriptional regulation in human chondrocytes.Exp Mol Med. 2016 Jun 24;48(6):e241. doi: 10.1038/emm.2016.47. Exp Mol Med. 2016. PMID: 27339399 Free PMC article.
-
Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1beta.Osteoarthritis Cartilage. 2008 Dec;16(12):1560-71. doi: 10.1016/j.joca.2008.04.027. Epub 2008 Jun 18. Osteoarthritis Cartilage. 2008. PMID: 18565769 Free PMC article.
-
Transcriptional regulation of the human iNOS gene by IL-1beta in endothelial cells.Mol Med. 2001 May;7(5):329-43. Mol Med. 2001. PMID: 11474579 Free PMC article.
Cited by
-
Molecular characterization of articular cartilage from young adults with femoroacetabular impingement.J Bone Joint Surg Am. 2013 Aug 21;95(16):1457-64. doi: 10.2106/JBJS.L.00497. J Bone Joint Surg Am. 2013. PMID: 23965695 Free PMC article.
-
Effects of CD14 macrophages and proinflammatory cytokines on chondrogenesis in osteoarthritic synovium-derived stem cells.Tissue Eng Part A. 2014 Oct;20(19-20):2680-91. doi: 10.1089/ten.TEA.2013.0656. Epub 2014 Jul 22. Tissue Eng Part A. 2014. PMID: 24806317 Free PMC article. Clinical Trial.
-
The inflammatory response in acyl-CoA oxidase 1 deficiency (pseudoneonatal adrenoleukodystrophy).Endocrinology. 2012 Jun;153(6):2568-75. doi: 10.1210/en.2012-1137. Epub 2012 Apr 16. Endocrinology. 2012. PMID: 22508517 Free PMC article.
-
Transcriptome Analysis and Gene Identification in the Pulmonary Artery of Broilers with Ascites Syndrome.PLoS One. 2016 Jun 8;11(6):e0156045. doi: 10.1371/journal.pone.0156045. eCollection 2016. PLoS One. 2016. PMID: 27275925 Free PMC article.
-
Methylmercury induces the expression of chemokine CCL4 via SRF activation in C17.2 mouse neural stem cells.Sci Rep. 2019 Mar 15;9(1):4631. doi: 10.1038/s41598-019-41127-y. Sci Rep. 2019. PMID: 30874621 Free PMC article.
References
-
- Gerard C., Rollins B. J. (2001) Nat. Immunol. 2, 108–115 - PubMed
-
- Acosta J. C., O'Loghlen A., Banito A., Guijarro M. V., Augert A., Raguz S., Fumagalli M., Da Costa M., Brown C., Popov N., Takatsu Y., Melamed J., d'Adda di Fagagna F., Bernard D., Hernando E., Gil J. (2008) Cell 133, 1006–1018 - PubMed
-
- Han J. H., Choi S. J., Kurihara N., Koide M., Oba Y., Roodman G. D. (2001) Blood 97, 3349–3353 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

