Structure and metal loading of a soluble periplasm cuproprotein
- PMID: 20702411
- PMCID: PMC2952252
- DOI: 10.1074/jbc.M110.153080
Structure and metal loading of a soluble periplasm cuproprotein
Abstract
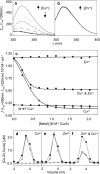
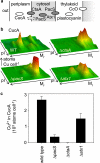
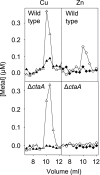
A copper-trafficking pathway was found to enable Cu(2+) occupancy of a soluble periplasm protein, CucA, even when competing Zn(2+) is abundant in the periplasm. Here, we solved the structure of CucA (a new cupin) and found that binding of Cu(2+), but not Zn(2+), quenches the fluorescence of Trp(165), which is adjacent to the metal site. Using this fluorescence probe, we established that CucA becomes partly occupied by Zn(2+) following exposure to equimolar Zn(2+) and Cu(2+). Cu(2+)-CucA is more thermodynamically stable than Zn(2+)-CucA but k((Zn→Cu)exchange) is slow, raising questions about how the periplasm contains solely the Cu(2+) form. We discovered that a copper-trafficking pathway involving two copper transporters (CtaA and PacS) and a metallochaperone (Atx1) is obligatory for Cu(2+)-CucA to accumulate in the periplasm. There was negligible CucA protein in the periplasm of ΔctaA cells, but the abundance of cucA transcripts was unaltered. Crucially, ΔctaA cells overaccumulate low M(r) copper complexes in the periplasm, and purified apoCucA can readily acquire Cu(2+) from ΔctaA periplasm extracts, but in vivo apoCucA fails to come into contact with these periplasmic copper pools. Instead, copper traffics via a cytoplasmic pathway that is coupled to CucA translocation to the periplasm.
Figures




References
-
- Nies D. H. (2007) in Molecular Microbiology of Heavy Metals (Nies D. H., Silver S. eds) pp. 117–142, Springer-Verlag, Heidelberg, Germany
-
- Tottey S., Waldron K. J., Firbank S. J., Reale B., Bessant C., Sato K., Cheek T. R., Gray J., Banfield M. J., Dennison C., Robinson N. J. (2008) Nature 455, 1138–1142 - PubMed
-
- Palmer T., Berks B. C. (2003) Microbiology 149, 547–556 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

