Foxm1 transcription factor is required for maintenance of pluripotency of P19 embryonal carcinoma cells
- PMID: 20702419
- PMCID: PMC3001083
- DOI: 10.1093/nar/gkq715
Foxm1 transcription factor is required for maintenance of pluripotency of P19 embryonal carcinoma cells
Abstract
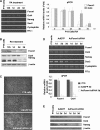
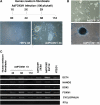
Transcription factor Foxm1 plays a critical role during embryonic development and its expression is repressed during retinoic acid (RA)-induced differentiation of pluripotent P19 embryonal carcinoma cells at the early stage, correlated with downregulation of expression of pluripotency markers. To study whether Foxm1 participates in the maintenance of pluripotency of stem cells, we knock down Foxm1 expression in P19 cells and identify that Oct4 are regulated directly by Foxm1. Knockdown of Foxm1 also results in spontaneous differentiation of P19 cells to mesodermal derivatives, such as muscle and adipose tissues. Maintaining Foxm1 expression prevents the downregulation of pluripotency-related transcription factors such as Oct4 and Nanog during P19 cell differentiation. Furthermore, overexpression of FOXM1 alone in RA-differentiated P19 cells (4 days) or human newborn fibroblasts restarts the expression of pluripotent genes Oct4, Nanog and Sox2. Together, our results suggest a critical involvement of Foxm1 in maintenance of stem cell pluripotency.
Figures



Similar articles
-
The miR-134 attenuates the expression of transcription factor FOXM1 during pluripotent NT2/D1 embryonal carcinoma cell differentiation.Exp Cell Res. 2015 Jan 15;330(2):442-450. doi: 10.1016/j.yexcr.2014.10.022. Epub 2014 Nov 4. Exp Cell Res. 2015. PMID: 25447206
-
Foxa1 contributes to the repression of Nanog expression by recruiting Grg3 during the differentiation of pluripotent P19 embryonal carcinoma cells.Exp Cell Res. 2014 Aug 15;326(2):326-35. doi: 10.1016/j.yexcr.2014.04.020. Epub 2014 May 5. Exp Cell Res. 2014. PMID: 24803390
-
Dual roles of Oct4 in the maintenance of mouse P19 embryonal carcinoma cells: as negative regulator of Wnt/β-catenin signaling and competence provider for Brachyury induction.Stem Cells Dev. 2011 Apr;20(4):621-33. doi: 10.1089/scd.2010.0209. Epub 2011 Jan 8. Stem Cells Dev. 2011. PMID: 21083502 Free PMC article.
-
Role of Oct4 in maintaining and regaining stem cell pluripotency.Stem Cell Res Ther. 2010 Dec 14;1(5):39. doi: 10.1186/scrt39. Stem Cell Res Ther. 2010. PMID: 21156086 Free PMC article. Review.
-
[OCT4 and NANOG are the key genes in the system of pluripotency maintenance in mammalian cells].Genetika. 2008 Dec;44(12):1589-608. Genetika. 2008. PMID: 19178078 Review. Russian.
Cited by
-
The transcription factor Foxm1 is essential for the quiescence and maintenance of hematopoietic stem cells.Nat Immunol. 2015 Aug;16(8):810-8. doi: 10.1038/ni.3204. Epub 2015 Jun 29. Nat Immunol. 2015. PMID: 26147687 Free PMC article.
-
TIN2 modulates FOXO1 mitochondrial shuttling to enhance oxidative stress-induced apoptosis in retinal pigment epithelium under hyperglycemia.Cell Death Differ. 2024 Nov;31(11):1487-1505. doi: 10.1038/s41418-024-01349-8. Epub 2024 Jul 30. Cell Death Differ. 2024. PMID: 39080375
-
FOXM1, MEK, and CDK4/6: New Targets for Malignant Peripheral Nerve Sheath Tumor Therapy.Int J Mol Sci. 2023 Sep 2;24(17):13596. doi: 10.3390/ijms241713596. Int J Mol Sci. 2023. PMID: 37686402 Free PMC article. Review.
-
A rare CHD5 haplotype and its interactions with environmental factors predicting hepatocellular carcinoma risk.BMC Cancer. 2018 Jun 15;18(1):658. doi: 10.1186/s12885-018-4551-y. BMC Cancer. 2018. PMID: 29907144 Free PMC article.
-
Loss of Forkhead box M1 promotes erythropoiesis through increased proliferation of erythroid progenitors.Haematologica. 2017 May;102(5):826-834. doi: 10.3324/haematol.2016.156257. Epub 2017 Feb 2. Haematologica. 2017. PMID: 28154085 Free PMC article.
References
-
- Martin G. Teratocarcinomas and mammalian embryogenesis. Science. 1980;209:768–776. - PubMed
-
- McBurney MW, Rogers BJ. Isolation of male embryonal carcinoma cells and their chromosome replication patterns. Dev. Biol. 1982;89:503–508. - PubMed
-
- Rossant J, McBurney MW. The developmental potential of a euploid male teratocarcinoma cell line after blastocyst injection. J. Embryol. Exp. Morphol. 1982;70:99–112. - PubMed
-
- McBurney MW, Jones-Villeneuve EM, Edwards MK, Anderson PJ. Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature. 1982;299:165–167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

