Response dynamics of phosphorelays suggest their potential utility in cell signalling
- PMID: 20702449
- PMCID: PMC3061117
- DOI: 10.1098/rsif.2010.0336
Response dynamics of phosphorelays suggest their potential utility in cell signalling
Abstract
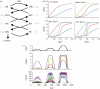
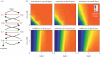
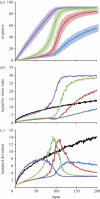
Phosphorelays are extended two-component signalling systems found in diverse bacteria, lower eukaryotes and plants. Only few of these systems are characterized, and we still lack a full understanding of their signalling abilities. Here, we aim to achieve a global understanding of phosphorelay signalling and its dynamical properties. We develop a generic model, allowing us to systematically analyse response dynamics under different assumptions. Using this model, we find that the steady-state concentration of phosphorylated protein at the final layer of a phosphorelay is a linearly increasing, but eventually saturating function of the input. In contrast, the intermediate layers can display ultrasensitivity. We find that such ultrasensitivity is a direct result of the phosphorelay biochemistry; shuttling of a single phosphate group from the first to the last layer. The response dynamics of the phosphorelay results in tolerance of cross-talk, especially when it occurs as cross-deactivation. Further, it leads to a high signal-to-noise ratio for the final layer. We find that a relay length of four, which is most commonly observed, acts as a saturating point for these dynamic properties. These findings suggest that phosphorelays could act as a mechanism to reduce noise and effects of cross-talk on the final layer of the relay and enforce its input-response relation to be linear. In addition, our analysis suggests that middle layers of phosphorelays could embed thresholds. We discuss the consequence of these findings in relation to why cells might use phosphorelays along with enzymatic kinase cascades.
Figures

Similar articles
-
Exact analysis of intrinsic qualitative features of phosphorelays using mathematical models.J Theor Biol. 2012 May 7;300:7-18. doi: 10.1016/j.jtbi.2012.01.007. Epub 2012 Jan 13. J Theor Biol. 2012. PMID: 22266661
-
Eukaryotic signal transduction via histidine-aspartate phosphorelay.J Cell Sci. 2000 Sep;113 ( Pt 18):3141-50. doi: 10.1242/jcs.113.18.3141. J Cell Sci. 2000. PMID: 10954413 Review.
-
In vitro analysis of His-Asp phosphorelays in Aspergillus nidulans: the first direct biochemical evidence for the existence of His-Asp phosphotransfer systems in filamentous fungi.Biosci Biotechnol Biochem. 2007 Oct;71(10):2493-502. doi: 10.1271/bbb.70292. Epub 2007 Oct 7. Biosci Biotechnol Biochem. 2007. PMID: 17928704
-
Two-component phosphorelays in fungal mitochondria and beyond.Mitochondrion. 2015 May;22:60-5. doi: 10.1016/j.mito.2015.03.003. Epub 2015 Apr 7. Mitochondrion. 2015. PMID: 25858273 Review.
-
Fungal histidine kinases.Sci STKE. 2001 Sep 4;2001(98):re1. doi: 10.1126/stke.2001.98.re1. Sci STKE. 2001. PMID: 11752677 Review.
Cited by
-
Systematic analysis of noise reduction properties of coupled and isolated feed-forward loops.PLoS Comput Biol. 2021 Dec 3;17(12):e1009622. doi: 10.1371/journal.pcbi.1009622. eCollection 2021 Dec. PLoS Comput Biol. 2021. PMID: 34860832 Free PMC article.
-
Phosphorelays provide tunable signal processing capabilities for the cell.PLoS Comput Biol. 2013;9(11):e1003322. doi: 10.1371/journal.pcbi.1003322. Epub 2013 Nov 7. PLoS Comput Biol. 2013. PMID: 24244132 Free PMC article.
-
Stochastic flux analysis of chemical reaction networks.BMC Syst Biol. 2013 Dec 7;7:133. doi: 10.1186/1752-0509-7-133. BMC Syst Biol. 2013. PMID: 24314153 Free PMC article.
-
Deciphering Parameter Sensitivity in the BvgAS Signal Transduction.PLoS One. 2016 Jan 26;11(1):e0147281. doi: 10.1371/journal.pone.0147281. eCollection 2016. PLoS One. 2016. PMID: 26812153 Free PMC article.
-
A Radical Reimagining of Fungal Two-Component Regulatory Systems.Trends Microbiol. 2021 Oct;29(10):883-893. doi: 10.1016/j.tim.2021.03.005. Epub 2021 Apr 12. Trends Microbiol. 2021. PMID: 33853736 Free PMC article. Review.
References
-
- Saito H. 2001. Histidine phosphorylation and two-component signaling in eukaryotic cells. Chem. Rev. 101, 2497–251010.1021/cr000243+ (doi:10.1021/cr000243+) - DOI - DOI - PubMed
-
- Thomason P., Kay R. 2000. Eukaryotic signal transduction via histidine-aspartate phosphorelay. J. Cell Sci. 113, 3141–3150 - PubMed
-
- West A. H., Stock A. M. 2001. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 26, 369–37610.1016/S0968-0004(01)01852-7 (doi:10.1016/S0968-0004(01)01852-7) - DOI - DOI - PubMed
-
- Cotter P. A., Jones A. M. 2003. Phosphorelay control of virulence gene expression in Bordetella. Trends Microbiol. 11, 367–37310.1016/S0966-842X(03)00156-2 (doi:10.1016/S0966-842X(03)00156-2) - DOI - DOI - PubMed
-
- Burbulys D., Trach K. A., Hoch J. A. 1991. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell 64, 545–55210.1016/0092-8674(91)90238-T (doi:10.1016/0092-8674(91)90238-T) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

