Response dynamics of phosphorelays suggest their potential utility in cell signalling
- PMID: 20702449
- PMCID: PMC3061117
- DOI: 10.1098/rsif.2010.0336
Response dynamics of phosphorelays suggest their potential utility in cell signalling
Abstract
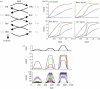
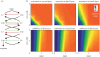
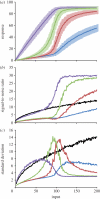
Phosphorelays are extended two-component signalling systems found in diverse bacteria, lower eukaryotes and plants. Only few of these systems are characterized, and we still lack a full understanding of their signalling abilities. Here, we aim to achieve a global understanding of phosphorelay signalling and its dynamical properties. We develop a generic model, allowing us to systematically analyse response dynamics under different assumptions. Using this model, we find that the steady-state concentration of phosphorylated protein at the final layer of a phosphorelay is a linearly increasing, but eventually saturating function of the input. In contrast, the intermediate layers can display ultrasensitivity. We find that such ultrasensitivity is a direct result of the phosphorelay biochemistry; shuttling of a single phosphate group from the first to the last layer. The response dynamics of the phosphorelay results in tolerance of cross-talk, especially when it occurs as cross-deactivation. Further, it leads to a high signal-to-noise ratio for the final layer. We find that a relay length of four, which is most commonly observed, acts as a saturating point for these dynamic properties. These findings suggest that phosphorelays could act as a mechanism to reduce noise and effects of cross-talk on the final layer of the relay and enforce its input-response relation to be linear. In addition, our analysis suggests that middle layers of phosphorelays could embed thresholds. We discuss the consequence of these findings in relation to why cells might use phosphorelays along with enzymatic kinase cascades.
Figures

References
-
- Saito H. 2001. Histidine phosphorylation and two-component signaling in eukaryotic cells. Chem. Rev. 101, 2497–251010.1021/cr000243+ (doi:10.1021/cr000243+) - DOI - DOI - PubMed
-
- Thomason P., Kay R. 2000. Eukaryotic signal transduction via histidine-aspartate phosphorelay. J. Cell Sci. 113, 3141–3150 - PubMed
-
- West A. H., Stock A. M. 2001. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 26, 369–37610.1016/S0968-0004(01)01852-7 (doi:10.1016/S0968-0004(01)01852-7) - DOI - DOI - PubMed
-
- Cotter P. A., Jones A. M. 2003. Phosphorelay control of virulence gene expression in Bordetella. Trends Microbiol. 11, 367–37310.1016/S0966-842X(03)00156-2 (doi:10.1016/S0966-842X(03)00156-2) - DOI - DOI - PubMed
-
- Burbulys D., Trach K. A., Hoch J. A. 1991. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell 64, 545–55210.1016/0092-8674(91)90238-T (doi:10.1016/0092-8674(91)90238-T) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
