The iron exporter ferroportin 1 is essential for development of the mouse embryo, forebrain patterning and neural tube closure
- PMID: 20702562
- PMCID: PMC2926957
- DOI: 10.1242/dev.048744
The iron exporter ferroportin 1 is essential for development of the mouse embryo, forebrain patterning and neural tube closure
Abstract
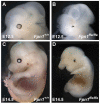
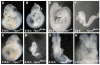
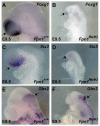
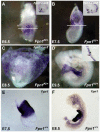
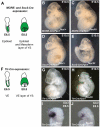
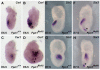
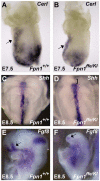
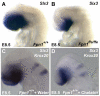
Neural tube defects (NTDs) are some of the most common birth defects observed in humans. The incidence of NTDs can be reduced by peri-conceptional folic acid supplementation alone and reduced even further by supplementation with folic acid plus a multivitamin. Here, we present evidence that iron maybe an important nutrient necessary for normal development of the neural tube. Following implantation of the mouse embryo, ferroportin 1 (Fpn1) is essential for the transport of iron from the mother to the fetus and is expressed in the visceral endoderm, yolk sac and placenta. The flatiron (ffe) mutant mouse line harbors a hypomorphic mutation in Fpn1 and we have created an allelic series of Fpn1 mutations that result in graded developmental defects. A null mutation in the Fpn1 gene is embryonic lethal before gastrulation, hypomorphic Fpn1(ffe/ffe) mutants exhibit NTDs consisting of exencephaly, spina bifida and forebrain truncations, while Fpn1(ffe/KI) mutants exhibit even more severe NTDs. We show that Fpn1 is not required in the embryo proper but rather in the extra-embryonic visceral endoderm. Our data indicate that loss of Fpn1 results in abnormal morphogenesis of the anterior visceral endoderm (AVE). Defects in the development of the forebrain in Fpn1 mutants are compounded by defects in multiple signaling centers required for maintenance of the forebrain, including the anterior definitive endoderm (ADE), anterior mesendoderm (AME) and anterior neural ridge (ANR). Finally, we demonstrate that this loss of forebrain maintenance is due in part to the iron deficiency that results from the absence of fully functional Fpn1.
Figures

Similar articles
-
High levels of iron supplementation prevents neural tube defects in the Fpn1ffe mouse model.Birth Defects Res. 2017 Jan 30;109(2):81-91. doi: 10.1002/bdra.23542. Birth Defects Res. 2017. PMID: 28008752 Free PMC article.
-
Maintenance of the specification of the anterior definitive endoderm and forebrain depends on the axial mesendoderm: a study using HNF3beta/Foxa2 conditional mutants.Dev Biol. 2002 Mar 1;243(1):20-33. doi: 10.1006/dbio.2001.0536. Dev Biol. 2002. PMID: 11846474
-
The zinc-finger protein CNBP is required for forebrain formation in the mouse.Development. 2003 Apr;130(7):1367-79. doi: 10.1242/dev.00349. Development. 2003. PMID: 12588852
-
Mini-review: toward understanding mechanisms of genetic neural tube defects in mice.Teratology. 1999 Nov;60(5):292-305. doi: 10.1002/(SICI)1096-9926(199911)60:5<292::AID-TERA10>3.0.CO;2-6. Teratology. 1999. PMID: 10525207 Review.
-
The visceral yolk sac endoderm provides for absorption of nutrients to the embryo during neurulation.Birth Defects Res A Clin Mol Teratol. 2010 Aug;88(8):593-600. doi: 10.1002/bdra.20705. Birth Defects Res A Clin Mol Teratol. 2010. PMID: 20672346 Review.
Cited by
-
Fetal Alcohol Spectrum Disorder and Iron Homeostasis.Nutrients. 2022 Oct 11;14(20):4223. doi: 10.3390/nu14204223. Nutrients. 2022. PMID: 36296909 Free PMC article. Review.
-
Ferredoxin reductase is critical for p53-dependent tumor suppression via iron regulatory protein 2.Genes Dev. 2017 Jun 15;31(12):1243-1256. doi: 10.1101/gad.299388.117. Epub 2017 Jul 26. Genes Dev. 2017. PMID: 28747430 Free PMC article.
-
Association of folate metabolism genes MTHFR and MTRR with multiple complex congenital malformation risk in Chinese population of Shanxi.Transl Pediatr. 2014 Jul;3(3):259-67. doi: 10.3978/j.issn.2224-4336.2014.07.10. Transl Pediatr. 2014. PMID: 26835343 Free PMC article.
-
Micronutrient imbalance and common phenotypes in neural tube defects.Genesis. 2021 Nov;59(11):e23455. doi: 10.1002/dvg.23455. Epub 2021 Oct 19. Genesis. 2021. PMID: 34665506 Free PMC article. Review.
-
Fetal liver hepcidin secures iron stores in utero.Blood. 2020 Sep 24;136(13):1549-1557. doi: 10.1182/blood.2019003907. Blood. 2020. PMID: 32542311 Free PMC article.
References
-
- Andrews N. C. (1999). Disorders of iron metabolism. New Engl. J. Med. 341, 1986-1995 - PubMed
-
- Ang S. L., Conlon R. A., Jin O., Rossant J. (1994). Positive and negative signals from mesoderm regulate the expression of mouse Otx2 in ectoderm explants. Development 120, 2979-2989 - PubMed
-
- Barnes J. D., Crosby J. L., Jones C. M., Wright C. V., Hogan B. L. (1994). Embryonic expression of Lim-1, the mouse homolog of Xenopus Xlim-1, suggests a role in lateral mesoderm differentiation and neurogenesis. Dev. Biol. 161, 168-178 - PubMed
-
- Belaoussoff M., Farrington S. M., Baron M. H. (1998). Hematopoietic induction and respecification of A-P identity by visceral endoderm signaling in the mouse embryo. Development 125, 5009-5018 - PubMed
-
- Belo J. A., Bouwmeester T., Leyns L., Kertesz N., Gallo M., Follettie M., De Robertis E. M. (1997). Cerberus-like is a secreted factor with neutralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech. Dev. 68, 45-57 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

