Sall1-dependent signals affect Wnt signaling and ureter tip fate to initiate kidney development
- PMID: 20702564
- PMCID: PMC2926958
- DOI: 10.1242/dev.037812
Sall1-dependent signals affect Wnt signaling and ureter tip fate to initiate kidney development
Abstract
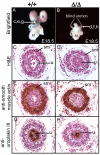
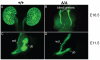
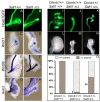
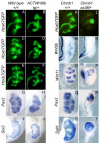
Development of the metanephric kidney depends on precise control of branching of the ureteric bud. Branching events represent terminal bifurcations that are thought to depend on unique patterns of gene expression in the tip compared with the stalk and are influenced by mesenchymal signals. The metanephric mesenchyme-derived signals that control gene expression at the ureteric bud tip are not well understood. In mouse Sall1 mutants, the ureteric bud grows out and invades the metanephric mesenchyme, but it fails to initiate branching despite tip-specific expression of Ret and Wnt11. The stalk-specific marker Wnt9b and the beta-catenin downstream target Axin2 are ectopically expressed in the mutant ureteric bud tips, suggesting that upregulated canonical Wnt signaling disrupts ureter branching in this mutant. In support of this hypothesis, ureter arrest is rescued by lowering beta-catenin levels in the Sall1 mutant and is phenocopied by ectopic expression of a stabilized beta-catenin in the ureteric bud. Furthermore, transgenic overexpression of Wnt9b in the ureteric bud causes reduced branching in multiple founder lines. These studies indicate that Sall1-dependent signals from the metanephric mesenchyme are required to modulate ureteric bud tip Wnt patterning in order to initiate branching.
Figures

Similar articles
-
Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis.Development. 2007 Jul;134(13):2397-405. doi: 10.1242/dev.02861. Epub 2007 May 23. Development. 2007. PMID: 17522159
-
Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene.Development. 2001 Dec;128(23):4747-56. doi: 10.1242/dev.128.23.4747. Development. 2001. PMID: 11731455
-
Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development.Development. 2003 Jul;130(14):3175-85. doi: 10.1242/dev.00520. Development. 2003. PMID: 12783789
-
GDNF/Ret signaling and the development of the kidney.Bioessays. 2006 Feb;28(2):117-27. doi: 10.1002/bies.20357. Bioessays. 2006. PMID: 16435290 Review.
-
The tip-top branching ureter.Curr Opin Cell Biol. 1997 Dec;9(6):877-84. doi: 10.1016/s0955-0674(97)80091-9. Curr Opin Cell Biol. 1997. PMID: 9425354 Review.
Cited by
-
SALL1 expression in acute myeloid leukemia.Oncotarget. 2017 Dec 15;9(7):7442-7452. doi: 10.18632/oncotarget.23448. eCollection 2018 Jan 26. Oncotarget. 2017. PMID: 29484122 Free PMC article.
-
Homozygous SALL1 mutation causes a novel multiple congenital anomaly-mental retardation syndrome.J Pediatr. 2013 Mar;162(3):612-7. doi: 10.1016/j.jpeds.2012.08.042. Epub 2012 Oct 12. J Pediatr. 2013. PMID: 23069192 Free PMC article.
-
Sall1 balances self-renewal and differentiation of renal progenitor cells.Development. 2014 Mar;141(5):1047-58. doi: 10.1242/dev.095851. Development. 2014. PMID: 24550112 Free PMC article.
-
FGF/EGF signaling regulates the renewal of early nephron progenitors during embryonic development.Development. 2011 Dec;138(23):5099-112. doi: 10.1242/dev.065995. Epub 2011 Oct 26. Development. 2011. PMID: 22031548 Free PMC article.
-
Disruption of Gen1 Causes Congenital Anomalies of the Kidney and Urinary Tract in Mice.Int J Biol Sci. 2018 Jan 1;14(1):10-20. doi: 10.7150/ijbs.22768. eCollection 2018. Int J Biol Sci. 2018. PMID: 29483821 Free PMC article.
References
-
- Basson M. A., Akbulut S., Watson-Johnson J., Simon R., Carroll T. J., Shakya R., Gross I., Martin G. R., Lufkin T., McMahon A. P., et al. (2005). Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev. Cell 8, 229-239 - PubMed
-
- Basson M. A., Watson-Johnson J., Shakya R., Akbulut S., Hyink D., Costantini F. D., Wilson P. D., Mason I. J., Licht J. D. (2006). Branching morphogenesis of the ureteric epithelium during kidney development is coordinated by the opposing functions of GDNF and Sprouty1. Dev. Biol. 299, 466-477 - PubMed
-
- Bohm J., Sustmann C., Wilhelm C., Kohlhase J. (2006). SALL4 is directly activated by TCF/LEF in the canonical Wnt signaling pathway. Biochem. Biophys. Res. Commun. 348, 898-907 - PubMed
-
- Bridgewater D., Cox B., Cain J., Lau A., Athaide V., Gill P. S., Kuure S., Sainio K., Rosenblum N. D. (2008). Canonical WNT/beta-catenin signaling is required for ureteric branching. Dev. Biol. 317, 83-94 - PubMed
-
- Bush K. T., Vaughn D. A., Li X., Rosenfeld M. G., Rose D. W., Mendoza S. A., Nigam S. K. (2006). Development and differentiation of the ureteric bud into the ureter in the absence of a kidney collecting system. Dev. Biol. 298, 571-584 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

