A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers
- PMID: 20702612
- PMCID: PMC3874864
- DOI: 10.1158/1078-0432.CCR-10-0733
A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers
Abstract
Purpose: Myeloid-derived suppressor cells (MDSC) accumulate in tumor-bearing hosts and are associated with immune suppression. To date, there have only been few studies that evaluate the direct effect of chemotherapeutic agents on MDSCs. Agents that inhibit MDSCs may be useful in the treatment of patients with various cancers.
Experimental design: We investigated the in vivo effects of docetaxel on immune function in 4T1-Neu mammary tumor-bearing mice to examine if a favorable immunomodulatory effect accompanies tumor suppression. Primary focus was on the differentiation status of MDSCs and their ability to modulate T-cell responses.
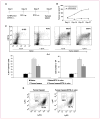
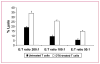
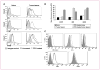
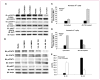
Results: Docetaxel administration significantly inhibited tumor growth in 4T1-Neu tumor-bearing mice and considerably decreased MDSC proportion in the spleen. The treatment also selectively increased CTL responses. Docetaxel-pretreated MDSCs cocultured with OT-II splenocytes in the presence of OVA(323-339) showed OT-II-specific CD4 activation and expansion in vitro. In characterizing the phenotype of MDSCs for M1 (CCR7) and M2 [mannose receptor (CD206)] markers, MDSCs from untreated tumor bearers were primarily MR(+) with few CCR7(+) cells. Docetaxel treatment polarized MDSCs toward an M1-like phenotype, resulting in 40% of MDSCs expressing CCR7 in vivo and in vitro, and macrophage differentiation markers such as MHC class II, CD11c, and CD86 were upregulated. Interestingly, docetaxel induced cell death selectively in MR(+) MDSCs while sparing the M1-like phenotype. Finally, inhibition of signal transducer and activator of transcription 3 may in part be responsible for the observed results.
Conclusions: These findings suggest potential clinical benefit for the addition of docetaxel to current immunotherapeutic protocols.
©2010 AACR.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


References
-
- Terabe M, Matsui S, Park JM, et al. Transforming growth factor-β production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–52. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

