Envelope-modified single-cycle simian immunodeficiency virus selectively enhances antibody responses and partially protects against repeated, low-dose vaginal challenge
- PMID: 20702641
- PMCID: PMC2950576
- DOI: 10.1128/JVI.00945-10
Envelope-modified single-cycle simian immunodeficiency virus selectively enhances antibody responses and partially protects against repeated, low-dose vaginal challenge
Abstract
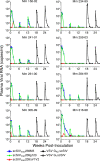
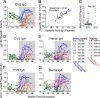
Immunization of rhesus macaques with strains of simian immunodeficiency virus (SIV) that are limited to a single cycle of infection elicits T-cell responses to multiple viral gene products and antibodies capable of neutralizing lab-adapted SIV, but not neutralization-resistant primary isolates of SIV. In an effort to improve upon the antibody responses, we immunized rhesus macaques with three strains of single-cycle SIV (scSIV) that express envelope glycoproteins modified to lack structural features thought to interfere with the development of neutralizing antibodies. These envelope-modified strains of scSIV lacked either five potential N-linked glycosylation sites in gp120, three potential N-linked glycosylation sites in gp41, or 100 amino acids in the V1V2 region of gp120. Three doses consisting of a mixture of the three envelope-modified strains of scSIV were administered on weeks 0, 6, and 12, followed by two booster inoculations with vesicular stomatitis virus (VSV) G trans-complemented scSIV on weeks 18 and 24. Although this immunization regimen did not elicit antibodies capable of detectably neutralizing SIV(mac)239 or SIV(mac)251(UCD), neutralizing antibody titers to the envelope-modified strains were selectively enhanced. Virus-specific antibodies and T cells were observed in the vaginal mucosa. After 20 weeks of repeated, low-dose vaginal challenge with SIV(mac)251(UCD), six of eight immunized animals versus six of six naïve controls became infected. Although immunization did not significantly reduce the likelihood of acquiring immunodeficiency virus infection, statistically significant reductions in peak and set point viral loads were observed in the immunized animals relative to the naïve control animals.
Figures




Similar articles
-
Analysis of Complement-Mediated Lysis of Simian Immunodeficiency Virus (SIV) and SIV-Infected Cells Reveals Sex Differences in Vaccine-Induced Immune Responses in Rhesus Macaques.J Virol. 2018 Sep 12;92(19):e00721-18. doi: 10.1128/JVI.00721-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30021899 Free PMC article.
-
Improved protection of rhesus macaques against intrarectal simian immunodeficiency virus SIV(mac251) challenge by a replication-competent Ad5hr-SIVenv/rev and Ad5hr-SIVgag recombinant priming/gp120 boosting regimen.J Virol. 2003 Aug;77(15):8354-65. doi: 10.1128/jvi.77.15.8354-8365.2003. J Virol. 2003. PMID: 12857905 Free PMC article.
-
Immunization with single-cycle SIV significantly reduces viral loads after an intravenous challenge with SIV(mac)239.PLoS Pathog. 2009 Jan;5(1):e1000272. doi: 10.1371/journal.ppat.1000272. Epub 2009 Jan 23. PLoS Pathog. 2009. PMID: 19165322 Free PMC article.
-
Targeted lymph-node immunization with whole inactivated simian immunodeficiency virus (SIV) or envelope and core subunit antigen vaccines does not reliably protect rhesus macaques from vaginal challenge with SIVmac251.AIDS. 1998 Jan 1;12(1):1-10. doi: 10.1097/00002030-199801000-00001. AIDS. 1998. PMID: 9456249 Free PMC article.
-
Antiviral CD8+ T cells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6.J Intern Med. 2009 Jan;265(1):67-77. doi: 10.1111/j.1365-2796.2008.02051.x. J Intern Med. 2009. PMID: 19093961 Free PMC article. Review.
Cited by
-
A novel assay for antibody-dependent cell-mediated cytotoxicity against HIV-1- or SIV-infected cells reveals incomplete overlap with antibodies measured by neutralization and binding assays.J Virol. 2012 Nov;86(22):12039-52. doi: 10.1128/JVI.01650-12. Epub 2012 Aug 29. J Virol. 2012. PMID: 22933282 Free PMC article.
-
Novel Compound Inhibitors of HIV-1NL4-3 Vpu.Viruses. 2022 Apr 15;14(4):817. doi: 10.3390/v14040817. Viruses. 2022. PMID: 35458546 Free PMC article.
-
Signatures in Simian Immunodeficiency Virus SIVsmE660 Envelope gp120 Are Associated with Mucosal Transmission but Not Vaccination Breakthrough in Rhesus Macaques.J Virol. 2015 Dec 16;90(4):1880-7. doi: 10.1128/JVI.02711-15. Print 2016 Feb 15. J Virol. 2015. PMID: 26676777 Free PMC article.
-
ADCC develops over time during persistent infection with live-attenuated SIV and is associated with complete protection against SIV(mac)251 challenge.PLoS Pathog. 2012;8(8):e1002890. doi: 10.1371/journal.ppat.1002890. Epub 2012 Aug 23. PLoS Pathog. 2012. PMID: 22927823 Free PMC article.
-
Mucosal priming with a replicating-vaccinia virus-based vaccine elicits protective immunity to simian immunodeficiency virus challenge in rhesus monkeys.J Virol. 2013 May;87(10):5669-77. doi: 10.1128/JVI.03247-12. Epub 2013 Mar 13. J Virol. 2013. PMID: 23487457 Free PMC article.
References
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Anonymous. 1996. Guide for the care and use of laboratory animals, p. 86-123. The Institute of Laboratory Animal Resources, National Research Council, Washington, DC.
-
- Baba, T. W., Y. S. Jeong, D. Pennick, R. Bronson, M. F. Greene, and R. M. Ruprecht. 1995. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science 267:1820-1825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

