Neuromodulation by GABA converts a relay into a coincidence detector
- PMID: 20702743
- PMCID: PMC2957460
- DOI: 10.1152/jn.00474.2010
Neuromodulation by GABA converts a relay into a coincidence detector
Abstract
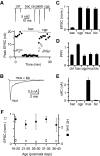
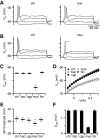
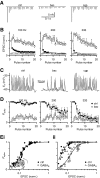
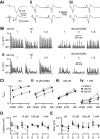
Modulation of synaptic strength by γ-aminobutyric acid receptors (GABARs) is a common feature in sensory pathways that contain relay cell types. However, the functional impact of these receptors on information processing is not clear. We considered this issue at bushy cells (BCs) in the cochlear nucleus, which relay auditory nerve (AN) activity to higher centers. BCs express GABA(A)Rs, and synaptic inputs to BCs express GABA(B)Rs. We tested the effects of GABAR activation on the relaying of AN activity using patch-clamp recordings in mature mouse brain slices at 34°C. GABA affected BC firing in response to trains of AN activity at concentrations as low as 10 μM. GABA(A)Rs reduced firing primarily late in high-frequency trains, whereas GABA(B)Rs reduced firing early and in low-frequency trains. BC firing was significantly restored when two converging AN inputs were activated simultaneously, with maximal effect over a window of <0.5 ms. Thus GABA could adjust the function of BCs, to suppress the relaying of individual inputs and require coincident activity of multiple inputs.
Figures



Similar articles
-
Globus pallidus neurons dynamically regulate the activity pattern of subthalamic nucleus neurons through the frequency-dependent activation of postsynaptic GABAA and GABAB receptors.J Neurosci. 2005 Jul 6;25(27):6304-15. doi: 10.1523/JNEUROSCI.0450-05.2005. J Neurosci. 2005. PMID: 16000620 Free PMC article.
-
Impact of synaptic depression on spike timing at the endbulb of Held.J Neurophysiol. 2009 Sep;102(3):1699-710. doi: 10.1152/jn.00072.2009. Epub 2009 Jul 8. J Neurophysiol. 2009. PMID: 19587324 Free PMC article.
-
Maturation of synaptic transmission at end-bulb synapses of the cochlear nucleus.J Neurosci. 2001 Dec 1;21(23):9487-98. doi: 10.1523/JNEUROSCI.21-23-09487.2001. J Neurosci. 2001. PMID: 11717383 Free PMC article.
-
Bridging the cleft at GABA synapses in the brain.Trends Neurosci. 1994 Dec;17(12):517-25. doi: 10.1016/0166-2236(94)90155-4. Trends Neurosci. 1994. PMID: 7532336 Review.
-
The role of GABA(B) receptors in the regulation of excitatory neurotransmission.Results Probl Cell Differ. 2008;44:87-98. doi: 10.1007/400_2007_038. Results Probl Cell Differ. 2008. PMID: 17549439 Review.
Cited by
-
Transmission of auditory sensory information decreases in rate and temporal precision at the endbulb of Held synapse during age-related hearing loss.J Neurophysiol. 2016 Dec 1;116(6):2695-2705. doi: 10.1152/jn.00472.2016. Epub 2016 Sep 28. J Neurophysiol. 2016. PMID: 27683884 Free PMC article.
-
Induction of synapse formation by de novo neurotransmitter synthesis.Nat Commun. 2022 Jun 1;13(1):3060. doi: 10.1038/s41467-022-30756-z. Nat Commun. 2022. PMID: 35650274 Free PMC article.
-
Cellular and synaptic specializations for sub-millisecond precision in the mammalian auditory brainstem.Front Cell Neurosci. 2025 May 19;19:1568506. doi: 10.3389/fncel.2025.1568506. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40458470 Free PMC article. Review.
-
Skipped-stimulus approach reveals that short-term plasticity dominates synaptic strength during ongoing activity.J Neurosci. 2015 May 27;35(21):8297-307. doi: 10.1523/JNEUROSCI.4299-14.2015. J Neurosci. 2015. PMID: 26019343 Free PMC article.
-
Time Course of Activity-Dependent Changes in Auditory Nerve Synapses Reveals Multiple Underlying Cellular Mechanisms.J Neurosci. 2022 Mar 23;42(12):2492-2502. doi: 10.1523/JNEUROSCI.1583-21.2022. Epub 2022 Feb 18. J Neurosci. 2022. PMID: 35181597 Free PMC article.
References
-
- Adams JC, Mugnaini E. Patterns of glutamate decarboxylase immunostaining in the feline cochlear nuclear complex studied with silver enhancement and electron microscopy. J Comp Neurol 262: 375–401, 1987. - PubMed
-
- Agmon-Snir H, Carr CE, Rinzel J. The role of dendrites in auditory coincidence detection. Nature 393: 268–272, 1998. - PubMed
-
- Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature 340: 153–156, 1989. - PubMed
-
- Bellingham MC, Walmsley B. A novel presynaptic inhibitory mechanism underlies paired pulse depression at a fast central synapse. Neuron 23: 159–170, 1999. - PubMed
-
- Benson CG, Potashner SJ. Retrograde transport of [3H]glycine from the cochlear nucleus to the superior olive in the guinea pig. J Comp Neurol 296: 415–426, 1990. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

