The B-cell tumor-associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor
- PMID: 20702778
- PMCID: PMC2996114
- DOI: 10.1182/blood-2010-05-283309
The B-cell tumor-associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor
Abstract
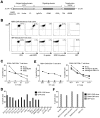
Monoclonal antibodies and T cells modified to express chimeric antigen receptors specific for B-cell lineage surface molecules such as CD20 exert antitumor activity in B-cell malignancies, but deplete normal B cells. The receptor tyrosine kinase-like orphan receptor 1 (ROR1) was identified as a highly expressed gene in B-cell chronic lymphocytic leukemia (B-CLL), but not normal B cells, suggesting it may serve as a tumor-specific target for therapy. We analyzed ROR1-expression in normal nonhematopoietic and hematopoietic cells including B-cell precursors, and in hematopoietic malignancies. ROR1 has characteristics of an oncofetal gene and is expressed in undifferentiated embryonic stem cells, B-CLL and mantle cell lymphoma, but not in major adult tissues apart from low levels in adipose tissue and at an early stage of B-cell development. We constructed a ROR1-specific chimeric antigen receptor that when expressed in T cells from healthy donors or CLL patients conferred specific recognition of primary B-CLL and mantle cell lymphoma, including rare drug effluxing chemotherapy resistant tumor cells that have been implicated in maintaining the malignancy, but not mature normal B cells. T-cell therapies targeting ROR1 may be effective in B-CLL and other ROR1-positive tumors. However, the expression of ROR1 on some normal tissues suggests the potential for toxi-city to subsets of normal cells.
Figures
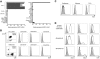



Comment in
-
RORing T cells target CLL and MCL.Blood. 2010 Nov 25;116(22):4387-8. doi: 10.1182/blood-2010-09-303388. Blood. 2010. PMID: 21109622 No abstract available.
References
-
- Maris MB, Sandmaier BM, Storer BE, et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104(12):3535–3542. - PubMed
-
- Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20(1):70–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

