Glucose uptake in rat extraocular muscles: effect of insulin and contractile activity
- PMID: 20702816
- PMCID: PMC3055760
- DOI: 10.1167/iovs.10-6081
Glucose uptake in rat extraocular muscles: effect of insulin and contractile activity
Abstract
Purpose: Extraocular muscles show specific adaptations to fulfill the metabolic demands imposed by their constant activity. One aspect that has not been explored is the availability of substrate for energy pathways in extraocular muscles. In limb muscles, glucose enters by way of GLUT1 and GLUT4 transporters in a process regulated by insulin and contractile activity to match metabolic supply to demand. This mechanism may not apply to extraocular muscles because their constant activity may require high basal (insulin- and activity-independent) glucose uptake. The authors tested the hypothesis that glucose uptake by extraocular muscles is not regulated by insulin or contractile activity.
Methods: Extraocular muscles from adult male Sprague-Dawley rats were incubated with 100 nM insulin or were electrically stimulated to contract (activity); glucose uptake was measured with 2-deoxy-d[1,2-(3)H]glucose. The contents of GLUT1, GLUT4, total and phosphorylated protein kinase B (Akt), phosphorylated AMP-activated protein kinase (AMPK), and glycogen synthase kinase 3 (GSK3) underwent Western blot analysis.
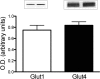
Results: Insulin and activity increased glucose uptake over the basal rate to 108% and 78%, respectively. GLUT1 and GLUT4 were detectable in extraocular muscles. Phosphorylated AKT/total AKT increased by twofold after insulin stimulation, but there was no change with activity. AMPK phosphorylation increased 35% with activity. Phosphorylated-GSK3/total GSK3 did not change with insulin or activity.
Conclusions: Glucose uptake in extraocular muscles is regulated by insulin and contractile activity. There is evidence of differences in the insulin signaling pathway that may explain the low glycogen content in these muscles.
Figures




Similar articles
-
Additive effect of contraction and insulin on glucose uptake and glycogen synthase in muscle with different glycogen contents.J Appl Physiol (1985). 2010 May;108(5):1106-15. doi: 10.1152/japplphysiol.00401.2009. Epub 2010 Feb 25. J Appl Physiol (1985). 2010. PMID: 20185632
-
Muscle glycogen inharmoniously regulates glycogen synthase activity, glucose uptake, and proximal insulin signaling.Am J Physiol Endocrinol Metab. 2006 Jan;290(1):E154-E162. doi: 10.1152/ajpendo.00330.2005. Epub 2005 Aug 23. Am J Physiol Endocrinol Metab. 2006. PMID: 16118249
-
Caffeine and theophylline block insulin-stimulated glucose uptake and PKB phosphorylation in rat skeletal muscles.Acta Physiol (Oxf). 2010 Sep;200(1):65-74. doi: 10.1111/j.1748-1716.2010.02103.x. Epub 2010 Feb 20. Acta Physiol (Oxf). 2010. PMID: 20180783
-
Prior treatment with the AMPK activator AICAR induces subsequently enhanced glucose uptake in isolated skeletal muscles from 24-month-old rats.Appl Physiol Nutr Metab. 2018 Aug;43(8):795-805. doi: 10.1139/apnm-2017-0858. Epub 2018 Mar 8. Appl Physiol Nutr Metab. 2018. PMID: 29518344 Free PMC article.
-
Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport.Appl Physiol Nutr Metab. 2007 Jun;32(3):557-66. doi: 10.1139/H07-026. Appl Physiol Nutr Metab. 2007. PMID: 17510697 Review.
Cited by
-
Rat diaphragm mitochondria have lower intrinsic respiratory rates than mitochondria in limb muscles.Am J Physiol Regul Integr Comp Physiol. 2011 Jun;300(6):R1311-5. doi: 10.1152/ajpregu.00203.2010. Epub 2011 Mar 9. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21389333 Free PMC article.
-
Myocardial insulin signaling and glucose transport are up-regulated in Goto-Kakizaki type 2 diabetic rats after ileal transposition.Obes Surg. 2012 Mar;22(3):493-501. doi: 10.1007/s11695-012-0604-5. Obes Surg. 2012. PMID: 22249887
References
-
- Porter JD. Commentary: extraocular muscle sparing in muscular dystrophy: a critical evaluation of potential protective mechanisms. Neuromuscul Disord. 1998;8:198–203 - PubMed
-
- Porter JD. Extraocular muscle: cellular adaptations for a diverse functional repertoire. Ann N Y Acad Sci. 2002;956:7–16 - PubMed
-
- Porter JD, Baker RS. Muscles of a different ‘color’: the unusual properties of the extraocular muscles may predispose or protect them in neurogenic and myogenic disease. Neurology. 1996;46:30–37 - PubMed
-
- Porter JD, Baker RS, Ragusa RJ, Brueckner JK. Extraocular muscles: basic and clinical aspects of structure and function. Surv Ophthalmol. 1995;39:451–484 - PubMed
-
- Sartore S, Mascarello F, Rowlerson A, et al. Fibre types in extraocular muscles: a new myosin isoform in the fast fibres. J Muscle Res Cell Motil. 1987;8:161–172 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

