Clinical and antiviral efficacy of an ophthalmic formulation of dexamethasone povidone-iodine in a rabbit model of adenoviral keratoconjunctivitis
- PMID: 20702820
- PMCID: PMC3053283
- DOI: 10.1167/iovs.10-5944
Clinical and antiviral efficacy of an ophthalmic formulation of dexamethasone povidone-iodine in a rabbit model of adenoviral keratoconjunctivitis
Abstract
Purpose: To determine the efficacy of a new formulation of topical dexamethasone 0.1%/povidone-iodine 0.4% (FST-100) in reducing clinical symptoms and infectious viral titers in a rabbit model of adenoviral keratoconjunctivitis.
Methods: Rabbit corneas were inoculated bilaterally with 2×10(6) plaque-forming-units (PFU) of adenovirus type 5 (Ad5) after corneal scarification. Animals were randomized 1:1:1:1 (five rabbits per group) to FST-100, 0.5% cidofovir, tobramycin/dexamethasone (Tobradex; Alcon Laboratories, Fort Worth, TX) ophthalmic suspension, and balanced salt solution (BSS; Alcon Laboratories). Treatment began 12 hours after viral inoculation and continued for 7 consecutive days. The eyes were clinically scored daily for scleral inflammation (injection), ocular neovascularization, eyelid inflammation (redness), friability of vasculature, inflammatory discharge (pus), and epiphora (excessive tearing). Eye swabs were collected daily before treatment for the duration of the study. Virus was eluted from the swabs and PFU determined by titration on human A549 cells, according to standard procedures.
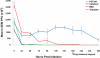
Results: The FST-100 treatment resulted in significantly lower clinical scores (P<0.05) than did the other treatments. The 0.5% cidofovir exhibited the most ocular toxicity compared with FST-100, tobramycin/dexamethasone, and balanced salt solution treatments. FST-100 and 0.5% cidofovir significantly (P<0.05) reduced viral titers compared with tobramycin/dexamethasone or balanced salt solution.
Conclusions: FST-100 was the most efficacious in minimizing the clinical symptoms of adenovirus infection in rabbit eyes. FST-100 and 0.5% cidofovir were both equally effective in reducing viral titers and decreasing the duration of viral shedding. By providing symptomatic relief in addition to reducing infectious virus titers, FST-100 should be a valuable addition to treatment of epidemic adenoviral keratoconjunctivitis.
Figures


References
-
- Schrauder A, Altmann D, Laude G, et al. Epidemic conjunctivitis in Germany. 2004. Eur Surveill. 2006;11:185–187 - PubMed
-
- Asencio-Durán M, Romero-Martín R, García-Martínez JR, et al. Nosocomial outbreak of epidemic keratoconjunctivitis in a neonatal intensive care unit (in Spanish). Arch Soc Esp Oftalmol. 2007;82:73–79 - PubMed
-
- Kumar NL, Black D, McClellan K. Daytime presentations to a metropolitan ophthalmic emergency department. Clin Exp Ophthalmol. 2005;33:586–592 - PubMed
-
- Ford E, Nelson KE, Warren D. Epidemiology of epidemic keratoconjunctivitis. Epidemiol Rev. 1987;9:244–261 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

