Biological activities of 7-dehydrocholesterol-derived oxysterols: implications for Smith-Lemli-Opitz syndrome
- PMID: 20702862
- PMCID: PMC2952566
- DOI: 10.1194/jlr.M009365
Biological activities of 7-dehydrocholesterol-derived oxysterols: implications for Smith-Lemli-Opitz syndrome
Abstract
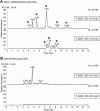
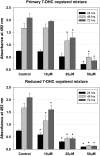
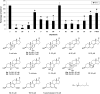
Smith-Lemli-Opitz syndrome (SLOS) is a metabolic and developmental disorder caused by mutations in the gene encoding the enzyme 7-dehydrocholesterol reductase (Dhcr7). This reductase catalyzes the last step in cholesterol biosynthesis, and levels of 7-dehydrocholesterol (7-DHC), the substrate for this enzyme, are elevated in SLOS patients as a result of this defect. Our group has previously shown that 7-DHC is extremely prone to free radical autoxidation, and we identified about a dozen different oxysterols formed from oxidation of 7-DHC. We report here that 7-DHC-derived oxysterols reduce cell viability in a dose- and time-dependent manner, some of the compounds showing activity at sub-micromolar concentrations. The reduction of cell survival is caused by a combination of reduced proliferation and induced differentiation of the Neuro2a cells. The complex 7-DHC oxysterol mixture added to control Neuro2a cells also triggers the gene expression changes that were previously identified in Dhcr7-deficient Neuro2a cells. Based on the identification of overlapping gene expression changes in Dhcr7-deficient and 7-DHC oxysterol-treated Neuro2a cells, we hypothesize that some of the pathophysiological findings in the mouse SLOS model and SLOS patients might be due to accumulated 7-DHC oxysterols.
Figures


References
-
- Porter N. A. 1986. Mechanisms for the autoxidation of polyunsaturated lipids. Acc. Chem. Res. 19: 262–270.
-
- Porter N. A., Caldwell S. E., Mills K. A. 1995. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 30: 277–290. - PubMed
-
- Yin H., Porter N. A. 2005. New insights regarding the autoxidation of polyunsaturated fatty acids. Antioxid. Redox Signal. 7: 170–184. - PubMed
-
- Porter N. A., Funk M. O., Gilmore D. W., Isaac R., Nixon J. 1976. The formation of cyclic peroxides from unsaturated hydroperoxides: models for prostaglandin biosynthesis. J. Am. Chem. Soc. 98: 6000–6005. - PubMed
-
- Porter N. A., Weber B. A., Weenen H., Khan J. A. 1980. Autoxidation of polyunsaturated lipids. Factors controlling the stereochemistry of product hydroperoxides. J. Am. Chem. Soc. 102: 5597–5601.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

