The use of divalent metal ions by type II topoisomerases
- PMID: 20703329
- PMCID: PMC2918885
- DOI: 10.1039/c003759a
The use of divalent metal ions by type II topoisomerases
Abstract
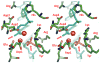
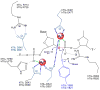
Type II topoisomerases are essential enzymes that regulate DNA under- and overwinding and remove knots and tangles from the genetic material. In order to carry out their critical physiological functions, these enzymes utilize a double-stranded DNA passage mechanism that requires them to generate a transient double-stranded break. Consequently, while necessary for cell survival, type II topoisomerases also have the capacity to fragment the genome. This feature of the prokaryotic and eukaryotic enzymes, respectively, is exploited to treat a variety of bacterial infections and cancers in humans. All type II topoisomerases require divalent metal ions for catalytic function. These metal ions function in two separate active sites and are necessary for the ATPase and DNA cleavage/ligation activities of the enzymes. ATPase activity is required for the strand passage process and utilizes the metal-dependent binding and hydrolysis of ATP to drive structural rearrangements in the protein. Both the DNA cleavage and ligation activities of type II topoisomerases require divalent metal ions and appear to utilize a novel variant of the canonical two-metal-ion phosphotransferase/hydrolase mechanism to facilitate these reactions. This article will focus primarily on eukaryotic type II topoisomerases and the roles of metal ions in the catalytic functions of these enzymes.
Keywords: ATP hydrolysis; DNA cleavage; DNA ligation; Topoisomerase IIα; divalent cation; divalent metal ion; topoisomerase II poisons.
Figures



Similar articles
-
Use of divalent metal ions in the dna cleavage reaction of human type II topoisomerases.Biochemistry. 2009 Mar 10;48(9):1862-9. doi: 10.1021/bi8023256. Biochemistry. 2009. PMID: 19222228 Free PMC article.
-
Metal ion interactions in the DNA cleavage/ligation active site of human topoisomerase IIalpha.Biochemistry. 2009 Sep 29;48(38):8940-7. doi: 10.1021/bi900875c. Biochemistry. 2009. PMID: 19697956 Free PMC article.
-
Topoisomerase II Poisons: Converting Essential Enzymes into Molecular Scissors.Biochemistry. 2021 Jun 1;60(21):1630-1641. doi: 10.1021/acs.biochem.1c00240. Epub 2021 May 19. Biochemistry. 2021. PMID: 34008964 Free PMC article. Review.
-
A novel and unified two-metal mechanism for DNA cleavage by type II and IA topoisomerases.Nature. 2010 Jun 3;465(7298):641-4. doi: 10.1038/nature08974. Nature. 2010. PMID: 20485342 Free PMC article.
-
The DNA cleavage reaction of topoisomerase II: wolf in sheep's clothing.Nucleic Acids Res. 2009 Feb;37(3):738-48. doi: 10.1093/nar/gkn937. Epub 2008 Nov 28. Nucleic Acids Res. 2009. PMID: 19042970 Free PMC article. Review.
Cited by
-
Topoisomerase II and leukemia.Ann N Y Acad Sci. 2014 Mar;1310(1):98-110. doi: 10.1111/nyas.12358. Epub 2014 Feb 3. Ann N Y Acad Sci. 2014. PMID: 24495080 Free PMC article. Review.
-
QnrS1 structure-activity relationships.J Antimicrob Chemother. 2014 Aug;69(8):2102-9. doi: 10.1093/jac/dku102. Epub 2014 Apr 11. J Antimicrob Chemother. 2014. PMID: 24729602 Free PMC article.
-
Use of divalent metal ions in the DNA cleavage reaction of topoisomerase IV.Nucleic Acids Res. 2011 Jun;39(11):4808-17. doi: 10.1093/nar/gkr018. Epub 2011 Feb 7. Nucleic Acids Res. 2011. PMID: 21300644 Free PMC article.
-
Prototype Foamy Virus Integrase Displays Unique Biochemical Activities among Retroviral Integrases.Biomolecules. 2021 Dec 20;11(12):1910. doi: 10.3390/biom11121910. Biomolecules. 2021. PMID: 34944553 Free PMC article.
-
Structural insights into the DNA topoisomerase II of the African swine fever virus.Nat Commun. 2024 May 30;15(1):4607. doi: 10.1038/s41467-024-49047-w. Nat Commun. 2024. PMID: 38816407 Free PMC article.
References
-
- Wang JC. Annu Rev Biochem. 1996;65:635–692. - PubMed
-
- Bates AD, Maxwell A. DNA Topology. Oxford University Press; New York: 2005.
-
- Espeli O, Marians KJ. Mol Microbiol. 2004;52:925–931. - PubMed
-
- Falaschi A, Abdurashidova G, Sandoval O, Radulescu S, Biamonti G, Riva S. Cell Cycle. 2007;6:1705–1712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

