A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer
- PMID: 20703455
- PMCID: PMC11030924
- DOI: 10.1007/s00262-010-0904-3
A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer
Abstract
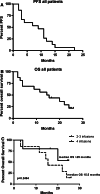
HLA-mismatched natural killer (NK) cells have shown efficacy in acute myeloid leukemia, and their adoptive transfer in patients with other malignancies has been proven safe. This phase I clinical trial was designed to evaluate safety (primary endpoint) and possible clinical efficacy (secondary endpoint) of repetitive administrations of allogeneic, in vitro activated and expanded NK cells along with chemotherapy in patients with advanced non-small cell lung cancer (NSCLC). Patients with unresectable, locally advanced/metastatic NSCLC receiving 1st/2nd line chemotherapy were eligible to receive 2-4 doses of activated NK cells from two relative donors. Donor's CD56(+) cells were cultured for 20-23 days with interleukin-15 (IL-15) and hydrocortisone (HC) and administered intravenously between chemotherapy cycles. Premedication with corticosteroids and/or H1 inhibitors was allowed. Sixteen patients (performance status 0-1) with adenocarcinoma (n = 13) or squamous cell carcinoma (n = 3) at stage IIIb (n = 5) or IV (n = 11) receiving 1st (n = 13) or 2nd (n = 3) line treatment were enrolled. Fifteen patients received 2-4 doses of allogeneic activated NK cells (0.2-29 × 10(6)/kg/dose, median 4.15 × 10(6)/kg/dose). No side effects (local or systemic) were observed. At a median 22-month follow-up (range, 16.5-26 months) 2 patients with partial response and 6 patients with disease stabilization were recorded. Median progression free survival and overall survival were 5.5 and 15 months, respectively. A 56% 1-year survival and a 19% 2-year survival were recorded. In conclusion, repetitive infusions of allogeneic, in vitro activated and expanded with IL-15/HC NK cells, in combination with chemotherapy are safe and potentially clinically effective.
Conflict of interest statement
None.
Figures

Similar articles
-
A trial of autologous ex vivo-expanded NK cell-enriched lymphocytes with docetaxel in patients with advanced non-small cell lung cancer as second- or third-line treatment: phase IIa study.Anticancer Res. 2013 May;33(5):2115-22. Anticancer Res. 2013. PMID: 23645763 Clinical Trial.
-
A phase I/II clinical trial on the efficacy and safety of NKT cells combined with gefitinib for advanced EGFR-mutated non-small-cell lung cancer.BMC Cancer. 2021 Jul 31;21(1):877. doi: 10.1186/s12885-021-08590-1. BMC Cancer. 2021. PMID: 34332557 Free PMC article. Clinical Trial.
-
Haploidentical Natural Killer Cells Infused before Allogeneic Stem Cell Transplantation for Myeloid Malignancies: A Phase I Trial.Biol Blood Marrow Transplant. 2016 Jul;22(7):1290-1298. doi: 10.1016/j.bbmt.2016.04.009. Epub 2016 Apr 16. Biol Blood Marrow Transplant. 2016. PMID: 27090958 Free PMC article. Clinical Trial.
-
Effectiveness and safety of chemotherapy with cytokine-induced killer cells in non-small cell lung cancer: A systematic review and meta-analysis of 32 randomized controlled trials.Cytotherapy. 2019 Feb;21(2):125-147. doi: 10.1016/j.jcyt.2018.10.011. Epub 2018 Dec 14. Cytotherapy. 2019. PMID: 30554868
-
Immune modulations during chemoimmunotherapy & novel vaccine strategies--in metastatic melanoma and non small-cell lung cancer.Dan Med J. 2013 Dec;60(12):B4774. Dan Med J. 2013. PMID: 24355457 Review.
Cited by
-
Enhancing a Natural Killer: Modification of NK Cells for Cancer Immunotherapy.Cells. 2021 Apr 29;10(5):1058. doi: 10.3390/cells10051058. Cells. 2021. PMID: 33946954 Free PMC article. Review.
-
Advances in the Lung Cancer Immunotherapy Approaches.Vaccines (Basel). 2022 Nov 19;10(11):1963. doi: 10.3390/vaccines10111963. Vaccines (Basel). 2022. PMID: 36423060 Free PMC article. Review.
-
NK cell subsets and dysfunction during viral infection: a new avenue for therapeutics?Front Immunol. 2023 Oct 19;14:1267774. doi: 10.3389/fimmu.2023.1267774. eCollection 2023. Front Immunol. 2023. PMID: 37928543 Free PMC article. Review.
-
Current progress in NK cell biology and NK cell-based cancer immunotherapy.Cancer Immunol Immunother. 2020 May;69(5):879-899. doi: 10.1007/s00262-020-02532-9. Epub 2020 Mar 4. Cancer Immunol Immunother. 2020. PMID: 32130453 Free PMC article. Review.
-
Retargeting IL-2 Signaling to NKG2D-Expressing Tumor-Infiltrating Leukocytes Improves Adoptive Transfer Immunotherapy.J Immunol. 2021 Jul 1;207(1):333-343. doi: 10.4049/jimmunol.2000926. Epub 2021 Jun 21. J Immunol. 2021. PMID: 34155069 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

