Molecular and structural analysis of mosaic variants of penicillin-binding protein 2 conferring decreased susceptibility to expanded-spectrum cephalosporins in Neisseria gonorrhoeae: role of epistatic mutations
- PMID: 20704258
- PMCID: PMC2939205
- DOI: 10.1021/bi101167x
Molecular and structural analysis of mosaic variants of penicillin-binding protein 2 conferring decreased susceptibility to expanded-spectrum cephalosporins in Neisseria gonorrhoeae: role of epistatic mutations
Abstract
Mutations in penicillin-binding protein 2 (PBP 2) encoded by mosaic penA alleles are crucial for intermediate resistance to the expanded-spectrum cephalosporins ceftriaxone and cefixime in Neisseria gonorrhoeae. Three of the ∼60 mutations present in mosaic alleles of penA, G545S, I312M, and V316T, have been reported to be responsible for increased resistance, especially to cefixime [Takahata, S., et al. (2006) Antimicrob. Agents Chemother. 50, 3638-3645]. However, we observed that the minimum inhibitory concentrations (MICs) of penicillin, ceftriaxone, and cefixime for a wild-type strain (FA19) containing a penA gene with these three mutations increased only 1.5-, 1.5-, and 3.5-fold, respectively. In contrast, when these three mutations in a mosaic penA allele (penA35) were reverted back to the wild type and the gene was transformed into FA19, the MICs of the three antibiotics were reduced to near wild-type levels. Thus, these three mutations display epistasis, in that their capacity to increase resistance to β-lactam antibiotics is dependent on the presence of other mutations in the mosaic alleles. We also identified an additional mutation, N512Y, that contributes to the decreased susceptibility to expanded-spectrum cephalosporins. Finally, we investigated the effects of a mutation (A501V) currently found only in nonmosaic penA alleles on decreased susceptibility to ceftriaxone and cefixime, with the expectation that this mutation may arise in mosaic alleles. Transfer of the mosaic penA35 allele containing an A501V mutation to FA6140, a chromosomally mediated penicillin-resistant isolate, increased the MICs of ceftriaxone (0.4 μg/mL) and cefixime (1.2 μg/mL) to levels above their respective break points. The proposed structural mechanisms of these mutations are discussed in light of the recently published structure of PBP 2.
Figures

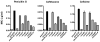


Similar articles
-
Identification of amino acids conferring high-level resistance to expanded-spectrum cephalosporins in the penA gene from Neisseria gonorrhoeae strain H041.Antimicrob Agents Chemother. 2013 Jul;57(7):3029-36. doi: 10.1128/AAC.00093-13. Epub 2013 Apr 15. Antimicrob Agents Chemother. 2013. PMID: 23587946 Free PMC article.
-
Alanine 501 Mutations in Penicillin-Binding Protein 2 from Neisseria gonorrhoeae: Structure, Mechanism, and Effects on Cephalosporin Resistance and Biological Fitness.Biochemistry. 2017 Feb 28;56(8):1140-1150. doi: 10.1021/acs.biochem.6b01030. Epub 2017 Feb 16. Biochemistry. 2017. PMID: 28145684 Free PMC article.
-
Analysis of amino acid sequences of penicillin-binding protein 2 in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime and ceftriaxone.J Infect Chemother. 2008 Jun;14(3):195-203. doi: 10.1007/s10156-008-0610-7. Epub 2008 Jun 24. J Infect Chemother. 2008. PMID: 18574654
-
Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime and ceftriaxone: association with genetic polymorphisms in penA, mtrR, porB1b, and ponA.Antimicrob Agents Chemother. 2007 Jun;51(6):2117-22. doi: 10.1128/AAC.01604-06. Epub 2007 Apr 9. Antimicrob Agents Chemother. 2007. PMID: 17420216 Free PMC article.
-
Affinity of β-Lactam Antibiotics for Neisseria gonorrhoeae Penicillin-Binding Protein 2 Having Wild, Cefixime-Reduced-Susceptible, and Cephalosporin (Ceftriaxone)-Resistant penA Alleles.Microb Drug Resist. 2024 Mar;30(3):141-146. doi: 10.1089/mdr.2023.0256. Epub 2024 Jan 12. Microb Drug Resist. 2024. PMID: 38215246
Cited by
-
Canary in the Coal Mine: How Resistance Surveillance in Commensals Could Help Curb the Spread of AMR in Pathogenic Neisseria.mBio. 2022 Oct 26;13(5):e0199122. doi: 10.1128/mbio.01991-22. Epub 2022 Sep 26. mBio. 2022. PMID: 36154280 Free PMC article. Review.
-
Antimicrobial susceptibility and genetic characteristics of Neisseria gonorrhoeae isolates from India, Pakistan and Bhutan in 2007-2011.BMC Infect Dis. 2013 Jan 24;13:35. doi: 10.1186/1471-2334-13-35. BMC Infect Dis. 2013. PMID: 23347339 Free PMC article.
-
Markedly Increasing Antibiotic Resistance and Dual Treatment of Neisseria gonorrhoeae Isolates in Guangdong, China, from 2013 to 2020.Antimicrob Agents Chemother. 2022 Apr 19;66(4):e0229421. doi: 10.1128/aac.02294-21. Epub 2022 Mar 29. Antimicrob Agents Chemother. 2022. PMID: 35345891 Free PMC article.
-
Antibiotic Targets in Gonococcal Cell Wall Metabolism.Antibiotics (Basel). 2018 Jul 21;7(3):64. doi: 10.3390/antibiotics7030064. Antibiotics (Basel). 2018. PMID: 30037076 Free PMC article. Review.
-
Antimicrobial resistance (AMR) and molecular characterization of Neisseria gonorrhoeae in Ghana, 2012-2015.PLoS One. 2019 Oct 10;14(10):e0223598. doi: 10.1371/journal.pone.0223598. eCollection 2019. PLoS One. 2019. PMID: 31600300 Free PMC article.
References
-
- CDC; Services, U. S. D. o. H. a. H. Sexually Transmitted Disease Surveillance 2007 Supplement, Gonococcal Isolate Surveillance Project (GISP) Annual Report 2007. Atlanta, Georgia: 2009.
-
- CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332–336. - PubMed
-
- Ameyama S, Onodera S, Takahata M, Minami S, Maki N, Endo K, Goto H, Suzuki H, Oishi Y. Mosaic-like structure of penicillin-binding protein 2 Gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob Agents Chemother. 2002;46:3744–3749. - PMC - PubMed
-
- Tapsall JW, Ndowa F, Lewis DA, Unemo M. Meeting the public health challenge of multidrug- and extensively drug-resistant Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2009;7:821–834. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

