Cytotoxic halogenated macrolides and modified peptides from the apratoxin-producing marine cyanobacterium Lyngbya bouillonii from Guam
- PMID: 20704304
- PMCID: PMC2965600
- DOI: 10.1021/np1004032
Cytotoxic halogenated macrolides and modified peptides from the apratoxin-producing marine cyanobacterium Lyngbya bouillonii from Guam
Abstract
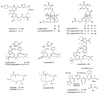
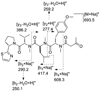
Collections of the marine cyanobacterium Lyngbya bouillonii from shallow patch reefs in Apra Harbor, Guam, afforded three hitherto undescribed analogues of the glycosidic macrolide lyngbyaloside, namely, 2-epi-lyngbyaloside (1) and the regioisomeric 18E- and 18Z-lyngbyalosides C (2 and 3). Concurrently we discovered two new analogues of the cytoskeletal actin-disrupting lyngbyabellins, 27-deoxylyngbyabellin A (4) and lyngbyabellin J (5), a novel macrolide of the laingolide family, laingolide B (6), and a linear modified peptide, lyngbyapeptin D (7), along with known lyngbyabellins A and B, lyngbyapeptin A, and lyngbyaloside. The structures of 1-7 were elucidated by a combination of NMR spectroscopic and mass spectrometric analysis. Compounds 1-6 were either brominated (1-3) or chlorinated (4-6), consistent with halogenation being a hallmark of many marine natural products. All extracts derived from these L. bouillonii collections were highly cytotoxic due to the presence of apratoxin A or apratoxin C. Compounds 1-5 showed weak to moderate cytotoxicity to HT29 colorectal adenocarcinoma and HeLa cervical carcinoma cells.
Figures

Similar articles
-
Apratoxin E, a cytotoxic peptolide from a guamanian collection of the marine cyanobacterium Lyngbya bouillonii.J Nat Prod. 2008 Jun;71(6):1113-6. doi: 10.1021/np700717s. Epub 2008 May 8. J Nat Prod. 2008. PMID: 18461997
-
Apratoxin D, a potent cytotoxic cyclodepsipeptide from papua new guinea collections of the marine cyanobacteria Lyngbya majuscula and Lyngbya sordida.J Nat Prod. 2008 Jun;71(6):1099-103. doi: 10.1021/np800121a. Epub 2008 Apr 30. J Nat Prod. 2008. PMID: 18444683
-
Lyngbyaloside B, a new glycoside macrolide from a Palauan marine cyanobacterium, Lyngbya sp.J Nat Prod. 2002 Dec;65(12):1945-8. doi: 10.1021/np0202879. J Nat Prod. 2002. PMID: 12502348
-
Malyngamide 3 and cocosamides A and B from the marine cyanobacterium Lyngbya majuscula from Cocos Lagoon, Guam.J Nat Prod. 2011 Apr 25;74(4):871-6. doi: 10.1021/np1008015. Epub 2011 Feb 22. J Nat Prod. 2011. PMID: 21341718 Free PMC article.
-
Continuing studies on the cyanobacterium Lyngbya sp.: isolation and structure determination of 15-norlyngbyapeptin A and lyngbyabellin D.J Nat Prod. 2003 May;66(5):595-8. doi: 10.1021/np030011g. J Nat Prod. 2003. PMID: 12762789
Cited by
-
Naturally Occurring Organohalogen Compounds-A Comprehensive Review.Prog Chem Org Nat Prod. 2023;121:1-546. doi: 10.1007/978-3-031-26629-4_1. Prog Chem Org Nat Prod. 2023. PMID: 37488466 Review.
-
Glycosylated Natural Products From Marine Microbes.Front Chem. 2020 Jan 10;7:879. doi: 10.3389/fchem.2019.00879. eCollection 2019. Front Chem. 2020. PMID: 31998682 Free PMC article. Review.
-
Sanctolide A, a 14-membered PK-NRP hybrid macrolide from the cultured cyanobacterium Oscillatoria sancta (SAG 74.79).Tetrahedron Lett. 2012 Jul 11;53(28):3563-3567. doi: 10.1016/j.tetlet.2012.04.136. Epub 2012 May 3. Tetrahedron Lett. 2012. PMID: 22711943 Free PMC article.
-
Biological targets and mechanisms of action of natural products from marine cyanobacteria.Nat Prod Rep. 2015 Mar;32(3):478-503. doi: 10.1039/c4np00104d. Nat Prod Rep. 2015. PMID: 25571978 Free PMC article. Review.
-
Total Synthesis and Structural Reassignment of Laingolide A.Mar Drugs. 2021 Apr 27;19(5):247. doi: 10.3390/md19050247. Mar Drugs. 2021. PMID: 33925490 Free PMC article.
References
-
- Tan LT. Phytochemistry. 2007;68:954–979. - PubMed
-
- Hoffmann L, Demoulin V. Belg. J. Bot. 1991;124:82–88.
-
- Luesch H. Ph.D. Thesis. Manoa, HI: University of Hawaii; 2002. The search for new anticancer drugs from marine cyanobacteria and investigations on the biosynthesis of cyanobacterial metabolites.
-
- Luesch H, Yoshida WY, Moore RE, Paul VJ, Corbett TH. J. Am. Chem. Soc. 2001;123:5418–5423. - PubMed
-
- Luesch H, Yoshida WY, Moore RE, Paul VJ, Mooberry SL. J. Nat. Prod. 2000;63:611–615. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

