Effective enrichment and mass spectrometry analysis of phosphopeptides using mesoporous metal oxide nanomaterials
- PMID: 20704311
- PMCID: PMC2936271
- DOI: 10.1021/ac100877a
Effective enrichment and mass spectrometry analysis of phosphopeptides using mesoporous metal oxide nanomaterials
Abstract
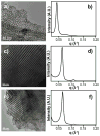
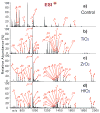
Mass spectrometry (MS)-based phosphoproteomics remains challenging due to the low abundance of phosphoproteins and substoichiometric phosphorylation. This demands better methods to effectively enrich phosphoproteins/peptides prior to MS analysis. We have previously communicated the first use of mesoporous zirconium dioxide (ZrO(2)) nanomaterials for effective phosphopeptide enrichment. Here, we present the full report including the synthesis, characterization, and application of mesoporous titanium dioxide (TiO(2)), ZrO(2), and hafnium dioxide (HfO(2)) in phosphopeptide enrichment and MS analysis. Mesoporous ZrO(2) and HfO(2) are demonstrated to be superior to TiO(2) for phosphopeptide enrichment from a complex mixture with high specificity (>99%), which could almost be considered as a "purification", mainly because of the extremely large active surface area of mesoporous nanomaterials. A single enrichment and Fourier transform MS analysis of phosphopeptides digested from a complex mixture containing 7% of alpha-casein identified 21 out of 22 phosphorylation sites for alpha-casein. Moreover, the mesoporous ZrO(2) and HfO(2) can be reused after a simple solution regeneration procedure with comparable enrichment performance to that of fresh materials. Mesoporous ZrO(2) and HfO(2) nanomaterials hold great promise for applications in MS-based phosphoproteomics.
Figures




Similar articles
-
Reactive landing of gas-phase ions as a tool for the fabrication of metal oxide surfaces for in situ phosphopeptide enrichment.J Am Soc Mass Spectrom. 2009 Jun;20(6):915-26. doi: 10.1016/j.jasms.2009.01.006. Epub 2009 Jan 22. J Am Soc Mass Spectrom. 2009. PMID: 19251440
-
Enrichment and analysis of phosphopeptides under different experimental conditions using titanium dioxide affinity chromatography and mass spectrometry.Rapid Commun Mass Spectrom. 2010 Jan;24(2):219-31. doi: 10.1002/rcm.4377. Rapid Commun Mass Spectrom. 2010. PMID: 20014058
-
Analysis of protein phosphorylation by monolithic extraction columns based on poly(divinylbenzene) containing embedded titanium dioxide and zirconium dioxide nano-powders.Proteomics. 2008 Nov;8(21):4593-602. doi: 10.1002/pmic.200800448. Proteomics. 2008. PMID: 18837466
-
Specific enrichment and direct detection of phosphopeptides on insoluble transition metal oxide particles in matrix-assisted laser desorption/ionization mass spectrometry applications.Eur J Mass Spectrom (Chichester). 2013;19(3):151-62. doi: 10.1255/ejms.1228. Eur J Mass Spectrom (Chichester). 2013. PMID: 24308196
-
Comparison of metal and metal oxide media for phosphopeptide enrichment prior to mass spectrometric analyses.J Am Soc Mass Spectrom. 2010 Oct;21(10):1649-59. doi: 10.1016/j.jasms.2010.06.005. Epub 2010 Jun 19. J Am Soc Mass Spectrom. 2010. PMID: 20634090 Free PMC article.
Cited by
-
Phosphorylation, but not alternative splicing or proteolytic degradation, is conserved in human and mouse cardiac troponin T.Biochemistry. 2011 Jul 12;50(27):6081-92. doi: 10.1021/bi2006256. Epub 2011 Jun 15. Biochemistry. 2011. PMID: 21639091 Free PMC article.
-
Amorphous TiO2 Nanotubes as a Platform for Highly Selective Phosphopeptide Enrichment.ACS Omega. 2019 Jul 15;4(7):12156-12166. doi: 10.1021/acsomega.9b00571. eCollection 2019 Jul 31. ACS Omega. 2019. PMID: 31460330 Free PMC article.
-
Specific enrichment of phosphoproteins using functionalized multivalent nanoparticles.J Am Chem Soc. 2015 Feb 25;137(7):2432-5. doi: 10.1021/ja511833y. Epub 2015 Feb 11. J Am Chem Soc. 2015. PMID: 25655481 Free PMC article.
-
Sequential enrichment with titania-coated magnetic mesoporous hollow silica microspheres and zirconium arsenate-modified magnetic nanoparticles for the study of phosphoproteome of HL60 cells.J Chromatogr A. 2014 Oct 24;1365:54-60. doi: 10.1016/j.chroma.2014.09.008. Epub 2014 Sep 11. J Chromatogr A. 2014. PMID: 25262027 Free PMC article.
-
Radical solutions: Principles and application of electron-based dissociation in mass spectrometry-based analysis of protein structure.Mass Spectrom Rev. 2018 Nov;37(6):750-771. doi: 10.1002/mas.21560. Epub 2018 Feb 9. Mass Spectrom Rev. 2018. PMID: 29425406 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

