p-Trifluoromethyldiazirinyl-etomidate: a potent photoreactive general anesthetic derivative of etomidate that is selective for ligand-gated cationic ion channels
- PMID: 20704351
- PMCID: PMC3117435
- DOI: 10.1021/jm100498u
p-Trifluoromethyldiazirinyl-etomidate: a potent photoreactive general anesthetic derivative of etomidate that is selective for ligand-gated cationic ion channels
Abstract
We synthesized the R- and S-enantiomers of ethyl 1-(1-(4-(3-((trifluoromethyl)-3H-diazirin-3-yl)phenyl)ethyl)-1H-imidazole-5-carboxylate (trifluoromethyldiazirinyl-etomidate), or TFD-etomidate, a novel photoactivable derivative of the stereoselective general anesthetic etomidate (R-(2-ethyl 1-(phenylethyl)-1H-imidazole-5-carboxylate)). Anesthetic potency was similar to etomidate's, but stereoselectivity was reversed and attenuated. Relative to etomidate, TFD-etomidate was a more potent inhibitor of the excitatory receptors, nAChR (nicotinic acetylcholine receptor) ((alpha1)(2)beta1delta1gamma1) and 5-HT(3A)R (serotonin type 3A receptor), causing significant inhibition at anesthetic concentrations. S- but not R-TFD-etomidate enhanced currents elicited from inhibitory alpha1beta2gamma2L GABA(A)Rs by low concentrations of GABA, but with a lower efficacy than R-etomidate, and site-directed mutagenesis suggests they act at different sites. [(3)H]TFD-etomidate photolabeled the alpha-subunit of the nAChR in a manner allosterically regulated by agonists and noncompetitive inhibitors. TFD-etomidate's novel pharmacology is unlike that of etomidate derivatives with photoactivable groups in the ester position, which behave like etomidate, suggesting that it will further enhance our understanding of anesthetic mechanisms.
Figures

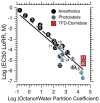
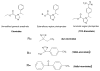

Similar articles
-
Synthesis of trifluoromethylaryl diazirine and benzophenone derivatives of etomidate that are potent general anesthetics and effective photolabels for probing sites on ligand-gated ion channels.J Med Chem. 2006 Aug 10;49(16):4818-25. doi: 10.1021/jm051207b. J Med Chem. 2006. PMID: 16884293
-
2-(3-Methyl-3H-diaziren-3-yl)ethyl 1-(1-phenylethyl)-1H-imidazole-5-carboxylate: a derivative of the stereoselective general anesthetic etomidate for photolabeling ligand-gated ion channels.J Med Chem. 2003 Mar 27;46(7):1257-65. doi: 10.1021/jm020465v. J Med Chem. 2003. PMID: 12646036
-
p-(4-Azipentyl)propofol: a potent photoreactive general anesthetic derivative of propofol.J Med Chem. 2011 Dec 8;54(23):8124-35. doi: 10.1021/jm200943f. Epub 2011 Nov 10. J Med Chem. 2011. PMID: 22029276 Free PMC article.
-
Anesthetic sites and allosteric mechanisms of action on Cys-loop ligand-gated ion channels.Can J Anaesth. 2011 Feb;58(2):191-205. doi: 10.1007/s12630-010-9419-9. Epub 2011 Jan 7. Can J Anaesth. 2011. PMID: 21213095 Free PMC article. Review.
-
GABA(A) receptors as molecular targets of general anesthetics: identification of binding sites provides clues to allosteric modulation.Can J Anaesth. 2011 Feb;58(2):206-15. doi: 10.1007/s12630-010-9429-7. Epub 2010 Dec 31. Can J Anaesth. 2011. PMID: 21194017 Free PMC article. Review.
Cited by
-
Shedding Light on Anesthetic Mechanisms: Application of Photoaffinity Ligands.Anesth Analg. 2016 Nov;123(5):1253-1262. doi: 10.1213/ANE.0000000000001365. Anesth Analg. 2016. PMID: 27464974 Free PMC article. Review.
-
Anesthetic Drug Discovery and Development: A Case Study of Novel Etomidate Analogs.Methods Enzymol. 2018;603:153-169. doi: 10.1016/bs.mie.2018.01.026. Epub 2018 Mar 2. Methods Enzymol. 2018. PMID: 29673523 Free PMC article.
-
Mechanisms revealed through general anesthetic photolabeling.Curr Anesthesiol Rep. 2014 Mar 1;4(1):57-66. doi: 10.1007/s40140-013-0040-7. Curr Anesthesiol Rep. 2014. PMID: 24563623 Free PMC article.
-
Mutations at beta N265 in γ-aminobutyric acid type A receptors alter both binding affinity and efficacy of potent anesthetics.PLoS One. 2014 Oct 27;9(10):e111470. doi: 10.1371/journal.pone.0111470. eCollection 2014. PLoS One. 2014. PMID: 25347186 Free PMC article.
-
Correction for Inhibition Leads to an Allosteric Co-Agonist Model for Pentobarbital Modulation and Activation of α1β3γ2L GABAA Receptors.PLoS One. 2016 Apr 25;11(4):e0154031. doi: 10.1371/journal.pone.0154031. eCollection 2016. PLoS One. 2016. PMID: 27110714 Free PMC article.
References
-
- Godefroi EF, Janssen PAJ, Van Der Eycken CAM, Van Heertum AHMT, Niemegeers CJE. DL-1-(1-Arylalkyl)imidazole-5-carboxylate Esters. A Novel Type of Hypnotic Agents. J Med Chem. 1965;8:220–223. - PubMed
-
- Tomlin SL, Jenkins A, Lieb WR, Franks NP. Stereoselective effects of etomidate optical isomers on gamma-aminobutyric acid type A receptors and animals. Anesthesiology. 1998;88:708–717. - PubMed
-
- Lambert JJ, Belelli D, Shepard S, Muntoni A-L, Pistis M, Peters JA. The GABA Receptor: An Important Locus for Intravenous Anæsthetic Action. In: Smith EB, Daniels S, editors. Gases in Medicine: Anæsthesia. Royal Society of Chemistry; Cambridge: 1998. pp. 121–137.
-
- Husain SS, Ziebell MR, Ruesch D, Hong F, Arevalo E, Kosterlitz JA, Olsen RW, Forman SA, Cohen JB, Miller KW. 2-(3-Methyl-3H-diaziren-3-yl)ethyl 1-(1-phenylethyl)-1H-imidazole-5-carboxylate: a derivative of the stereoselective general anesthetic etomidate for photolabeling ligand-gated ion channels. J Med Chem. 2003;46:1257–1265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

