A novel TMPRSS6 mutation that prevents protease auto-activation causes IRIDA
- PMID: 20704562
- PMCID: PMC2958558
- DOI: 10.1042/BJ20100668
A novel TMPRSS6 mutation that prevents protease auto-activation causes IRIDA
Abstract
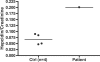
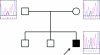
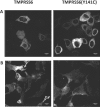
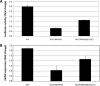
IRIDA (iron-refractory iron-deficiency anaemia) is a rare autosomal-recessive disorder hallmarked by hypochromic microcytic anaemia, low transferrin saturation and high levels of the iron-regulated hormone hepcidin. The disease is caused by mutations in the transmembrane serine protease TMPRSS6 (transmembrane protease serine 6) that prevent inactivation of HJV (haemojuvelin), an activator of hepcidin transcription. In the present paper, we describe a patient with IRIDA who carries a novel mutation (Y141C) in the SEA domain of the TMPRSS6 gene. Functional characterization of the TMPRSS6(Y141C) mutant protein in cultured cells showed that it localizes to similar subcellular compartments as wild-type TMPRSS6 and binds HJV, but fails to auto-catalytically activate itself. As a consequence, hepcidin mRNA expression is increased, causing the clinical symptoms observed in this IRIDA patient. The present study provides important mechanistic insight into how TMPRSS6 is activated.
Figures



References
-
- Buchanan G. R., Sheehan R. G. Malabsorption and defective utilization of iron in three siblings. J. Pediatr. 1981;98:723–728. - PubMed
-
- Melis M. A., Cau M., Congiu R., Sole G., Barella S., Cao A., Westerman M., Cazzola M., Galanello R. A mutation in the TMPRSS6 gene, encoding a transmembrane serine protease that suppresses hepcidin production, in familial iron deficiency anemia refractory to oral iron. Haematologica. 2008;93:1473–1479. - PubMed
-
- Pearson H. A., Lukens J. N. Ferrokinetics in the syndrome of familial hypoferremic microcytic anemia with iron malabsorption. J. Pediatr. Hematol. Oncol. 1999;21:412–417. - PubMed
-
- Brown A. C., Lutton J. D., Pearson H. A., Nelson J. C., Levere R. D., Abraham N. G. Heme metabolism and in vitro erythropoiesis in anemia associated with hypochromic microcytosis. Am. J. Hematol. 1988;27:1–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

