Decoding glutamate receptor activation by the Ca2+ sensor protein hippocalcin in rat hippocampal neurons
- PMID: 20704590
- PMCID: PMC3069492
- DOI: 10.1111/j.1460-9568.2010.07303.x
Decoding glutamate receptor activation by the Ca2+ sensor protein hippocalcin in rat hippocampal neurons
Abstract
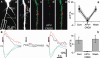
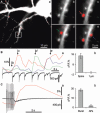
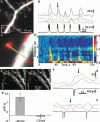
Hippocalcin is a Ca(2+)-binding protein that belongs to a family of neuronal Ca(2+)sensors and is a key mediator of many cellular functions including synaptic plasticity and learning. However, the molecular mechanisms involved in hippocalcin signalling remain illusive. Here we studied whether glutamate receptor activation induced by locally applied or synaptically released glutamate can be decoded by hippocalcin translocation. Local AMPA receptor activation resulted in fast hippocalcin-YFP translocation to specific sites within a dendritic tree mainly due to AMPA receptor-dependent depolarization and following Ca(2+)influx via voltage-operated calcium channels. Short local NMDA receptor activation induced fast hippocalcin-YFP translocation in a dendritic shaft at the application site due to direct Ca(2+)influx via NMDA receptor channels. Intrinsic network bursting produced hippocalcin-YFP translocation to a set of dendritic spines when they were subjected to several successive synaptic vesicle releases during a given burst whereas no translocation to spines was observed in response to a single synaptic vesicle release and to back-propagating action potentials. The translocation to spines required Ca(2+)influx via synaptic NMDA receptors in which Mg(2+) block is relieved by postsynaptic depolarization. This synaptic translocation was restricted to spine heads and even closely (within 1-2 microm) located spines on the same dendritic branch signalled independently. Thus, we conclude that hippocalcin may differentially decode various spatiotemporal patterns of glutamate receptor activation into site- and time-specific translocation to its targets. Hippocalcin also possesses an ability to produce local signalling at the single synaptic level providing a molecular mechanism for homosynaptic plasticity.
Figures




References
-
- Ames JB, Ishima R, Tanaka T, Gordon JI, Stryer L, Ikura M. Molecular mechanics of calcium-myristoyl switches. Nature. 1997;389:198–202. - PubMed
-
- Bardo S, Cavazzini MG, Emptage N. The role of the endoplasmic reticulum Ca2+store in the plasticity of central neurons. Trends Pharmacol. Sci. 2006;27:78–84. - PubMed
-
- Bayley P, Ahlstrom P, Martin SR, Forsen S. The kinetics of calcium binding to calmodulin: Quin 2 and ANS stopped-flow fluorescence studies. Biochem.Biophys.Res.Commun. 1984;120:185–191. - PubMed
-
- Ben Ari Y. Developing networks play a similar melody. Trends Neurosci. 2001;24:353–360. - PubMed
-
- Bezprozvanny I. The inositol 1,4,5-trisphosphate receptors. Cell Calcium. 2005;38:261–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

