A randomised controlled trial to evaluate the efficacy of a 6 month dietary and physical activity intervention for prostate cancer patients receiving androgen deprivation therapy
- PMID: 20704726
- PMCID: PMC2925820
- DOI: 10.1186/1745-6215-11-86
A randomised controlled trial to evaluate the efficacy of a 6 month dietary and physical activity intervention for prostate cancer patients receiving androgen deprivation therapy
Abstract
Background: Treatment with Androgen Deprivation Therapy (ADT) for prostate cancer is associated with changes in body composition including increased fat and decreased lean mass; increased fatigue, and a reduction in quality of life. No study to date has evaluated the effect of dietary and physical activity modification on the side-effects related to ADT. The aim of this study is to evaluate the efficacy of a 6-month dietary and physical activity intervention for prostate cancer survivors receiving ADT to minimise the changes in body composition, fatigue and quality of life, typically associated with ADT.
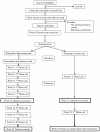
Methods: Men are recruited to this study if their treatment plan is to receive ADT for at least 6 months. Men who are randomised to the intervention arm receive a home-based tailored intervention to meet the following guidelines a) > or = 5 servings vegetables and fruits/day; b) 30%-35% of total energy from fat, and < 10% energy from saturated fat/day; c) 10% of energy from polyunsaturated fat/day; d) limited consumption of processed meats; e) 25-35 gm of fibre/day; f) alcoholic drinks < or = 28 units/week; g) limited intake of foods high in salt and/or sugar. They are also encouraged to include at least 30 minutes of brisk walking, 5 or more days per week. The primary outcomes are change in body composition, fatigue and quality of life scores. Secondary outcomes include dietary intake, physical activity and perceived stress. Baseline information collected includes: socio-economic status, treatment duration, perceived social support and health status, family history of cancer, co-morbidities, medication and supplement use, barriers to change, and readiness to change their health behaviour. Data for the primary and secondary outcomes will be collected at baseline, 3 and 6 months from 47 intervention and 47 control patients.
Discussion: The results of this study will provide detailed information on diet and physical activity levels in prostate cancer patients treated with ADT and will test the feasibility and efficacy of a diet and physical activity intervention which could provide essential information to develop guidelines for prostate cancer patients to minimise the side effects related to ADT.
Trial registration: ISRCTN trial number ISCRTN75282423.
Figures
References
-
- Cancer Research UK. Prostate cancer statistics - Key Facts. 2009. http://info.cancerresearchuk.org/cancerstats/types/prostate/
-
- Cohen SP, Jaskulsky SR. Prostate cancer Tx. Therapeutic options based on tumor grade, life expectancy, and patient preferences. Geriatrics. 2001;56:39–48. - PubMed
-
- Hussain S, Gunnell D, Donovan J, McPhail S, Hamdy F, Neal D, Albertsen P, Verne J, Stephens P, Trotter C, Martin RM. Secular trends in prostate cancer mortality, incidence and treatment: England and Wales, 1975-2004. BJU Int. 2008;101:547–555. doi: 10.1111/j.1464-410X.2007.07338.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical