Phospholipase D2-dependent inhibition of the nuclear hormone receptor PPARgamma by cyclic phosphatidic acid
- PMID: 20705243
- PMCID: PMC3446787
- DOI: 10.1016/j.molcel.2010.07.022
Phospholipase D2-dependent inhibition of the nuclear hormone receptor PPARgamma by cyclic phosphatidic acid
Abstract
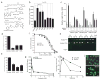
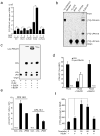
Cyclic phosphatidic acid (1-acyl-2,3-cyclic-glycerophosphate, CPA), one of nature's simplest phospholipids, is found in cells from slime mold to humans and has a largely unknown function. We find here that CPA is generated in mammalian cells in a stimulus-coupled manner by phospholipase D2 (PLD2) and binds to and inhibits the nuclear hormone receptor PPARgamma with nanomolar affinity and high specificity through stabilizing its interaction with the corepressor SMRT. CPA production inhibits the PPARgamma target-gene transcription that normally drives adipocytic differentiation of 3T3-L1 cells, lipid accumulation in RAW264.7 cells and primary mouse macrophages, and arterial wall remodeling in a rat model in vivo. Inhibition of PLD2 by shRNA, a dominant-negative mutant, or a small molecule inhibitor blocks CPA production and relieves PPARgamma inhibition. We conclude that CPA is a second messenger and a physiological inhibitor of PPARgamma, revealing that PPARgamma is regulated by endogenous agonists as well as by antagonists.
Copyright 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest statement: GT is a founder of RxBio Inc.
Figures





References
-
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. - PubMed
-
- Boullier A, Gillotte KL, Horkko S, Green SR, Friedman P, Dennis EA, Witztum JL, Steinberg D, Quehenberger O. The binding of oxidized low density lipoprotein to mouse CD36 is mediated in part by oxidized phospholipids that are associated with both the lipid and protein moieties of the lipoprotein. J Biol Chem. 2000;275:9163–9169. - PubMed
-
- Breen DM, Chan KK, Dhaliwall JK, Ward MR, Al Koudsi N, Lam L, De Souza M, Ghanim H, Dandona P, Stewart DJ, et al. Insulin increases reendothelialization and inhibits cell migration and neointimal growth after arterial injury. Arterioscler Thromb Vasc Biol. 2009;29:1060–1066. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

