Systematic review highlights difficulty interpreting diverse clinical outcomes in abnormal uterine bleeding trials
- PMID: 20705427
- PMCID: PMC3057176
- DOI: 10.1016/j.jclinepi.2010.03.017
Systematic review highlights difficulty interpreting diverse clinical outcomes in abnormal uterine bleeding trials
Abstract
Objectives: (1) To systematically collect and organize into clinical categories all outcomes reported in trials for abnormal uterine bleeding (AUB); (2) to rank the importance of outcomes for patient decision making; and (3) to improve future comparisons of effects in trials of AUB interventions.
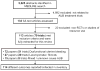
Study design and setting: Systematic review of English-language randomized controlled trials of AUB treatments in MEDLINE from 1950 to June 2008. All outcomes and definitions were extracted and organized into major outcome categories by an expert group. Each outcome was ranked "critically important," "important," or "not important" for informing patients' choices.
Results: One hundred thirteen articles from 79 trials met the criteria. One hundred fourteen different outcomes were identified, only 15 (13%) of which were ranked as critically important and 29 (25%) as important. Outcomes were grouped into eight categories: (1) bleeding; (2) quality of life; (3) pain; (4) sexual health; (5) patient satisfaction; (6) bulk-related complaints; (7) need for subsequent surgical treatment; and (8) adverse events.
Conclusion: To improve the quality, consistency, and utility of future AUB trials, we recommend assessing a limited number of clinical outcomes for bleeding, disease-specific quality of life, pain, sexual health, and bulk-related symptoms both before and after treatment and reporting satisfaction and adverse events. Further development of validated patient-based outcome measures and the standardization of outcome reporting are needed.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
References
-
- van Dongen H, van de Merwe AG, de Kroon CD, Jansen FW. Diagnostic hysteroscopy in abnormal uterine bleeding: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2009;16:47–51. - PubMed
-
- Côté I, Jacobs P, Cumming DC. Use of health services associated with increased menstrual loss in the United States. Am J Obstet Gynecol. 2003;188:343–8. - PubMed
-
- Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health. 2007;10:183–94. - PubMed
-
- Haynes P, Hodgson H, Anderson AB, Turnbull AC. Measurement of menstrual blood loss in patients complaining of menorrhagia. Br J Obstet Gynaecol. 1977;84:763–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous