Effect of trehalose on the properties of mutant {gamma}PKC, which causes spinocerebellar ataxia type 14, in neuronal cell lines and cultured Purkinje cells
- PMID: 20705605
- PMCID: PMC2963337
- DOI: 10.1074/jbc.M110.146704
Effect of trehalose on the properties of mutant {gamma}PKC, which causes spinocerebellar ataxia type 14, in neuronal cell lines and cultured Purkinje cells
Abstract
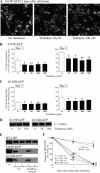
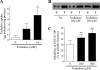
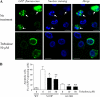
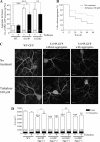
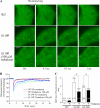
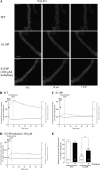
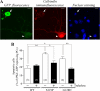
Several missense mutations in the protein kinase Cγ (γPKC) gene have been found to cause spinocerebellar ataxia type 14 (SCA14), an autosomal dominant neurodegenerative disease. We previously demonstrated that the mutant γPKC found in SCA14 is susceptible to aggregation, which induces apoptotic cell death. The disaccharide trehalose has been reported to inhibit aggregate formation and to alleviate symptoms in cellular and animal models of Huntington disease, Alzheimer disease, and prion disease. Here, we show that trehalose can be incorporated into SH-SY5Y cells and reduces the aggregation of mutant γPKC-GFP, thereby inhibiting apoptotic cell death in SH-SY5Y cells and primary cultured Purkinje cells (PCs). Trehalose acts by directly stabilizing the conformation of mutant γPKC without affecting protein turnover. Trehalose was also found to alleviate the improper development of dendrites in PCs expressing mutant γPKC-GFP without aggregates but not in PCs with aggregates. In PCs without aggregates, trehalose improves the mobility and translocation of mutant γPKC-GFP, probably by inhibiting oligomerization and thereby alleviating the improper development of dendrites. These results suggest that trehalose counteracts various cellular dysfunctions that are triggered by mutant γPKC in both neuronal cell lines and primary cultured PCs by inhibiting oligomerization and aggregation of mutant γPKC.
Figures

Similar articles
-
Elucidation of the molecular mechanism and exploration of novel therapeutics for spinocerebellar ataxia caused by mutant protein kinase Cγ.J Pharmacol Sci. 2011;116(3):239-47. doi: 10.1254/jphs.11r04cp. Epub 2011 Jun 10. J Pharmacol Sci. 2011. PMID: 21666345 Review.
-
Congo red, an amyloid-inhibiting compound, alleviates various types of cellular dysfunction triggered by mutant protein kinase cγ that causes spinocerebellar ataxia type 14 (SCA14) by inhibiting oligomerization and aggregation.J Pharmacol Sci. 2010;114(2):206-16. doi: 10.1254/jphs.10170fp. J Pharmacol Sci. 2010. PMID: 20938103
-
Mutant gammaPKC found in spinocerebellar ataxia type 14 induces aggregate-independent maldevelopment of dendrites in primary cultured Purkinje cells.Neurobiol Dis. 2009 Feb;33(2):260-73. doi: 10.1016/j.nbd.2008.10.013. Epub 2008 Nov 8. Neurobiol Dis. 2009. PMID: 19041943
-
Mutant protein kinase Cgamma found in spinocerebellar ataxia type 14 is susceptible to aggregation and causes cell death.J Biol Chem. 2005 Aug 12;280(32):29096-106. doi: 10.1074/jbc.M501716200. Epub 2005 Jun 17. J Biol Chem. 2005. PMID: 15964845
-
Calcium Signaling, PKC Gamma, IP3R1 and CAR8 Link Spinocerebellar Ataxias and Purkinje Cell Dendritic Development.Curr Neuropharmacol. 2018 Jan 30;16(2):151-159. doi: 10.2174/1570159X15666170529104000. Curr Neuropharmacol. 2018. PMID: 28554312 Free PMC article. Review.
Cited by
-
Pharmacological induction of heat shock proteins ameliorates toxicity of mutant PKCγ in spinocerebellar ataxia type 14.J Biol Chem. 2018 Sep 21;293(38):14758-14774. doi: 10.1074/jbc.RA118.002913. Epub 2018 Aug 9. J Biol Chem. 2018. PMID: 30093405 Free PMC article.
-
Trehalose reduces retinal degeneration, neuroinflammation and storage burden caused by a lysosomal hydrolase deficiency.Autophagy. 2018;14(8):1419-1434. doi: 10.1080/15548627.2018.1474313. Epub 2018 Jul 23. Autophagy. 2018. PMID: 29916295 Free PMC article.
-
Trehalose attenuates the gait ataxia and gliosis of spinocerebellar ataxia type 17 mice.Neurochem Res. 2015 Apr;40(4):800-10. doi: 10.1007/s11064-015-1530-4. Epub 2015 Feb 12. Neurochem Res. 2015. PMID: 25672822
-
Testosterone promotes GPX5 expression of goat epididymal epithelial cells cultured in vitro.In Vitro Cell Dev Biol Anim. 2019 Oct;55(9):677-685. doi: 10.1007/s11626-019-00391-y. Epub 2019 Aug 19. In Vitro Cell Dev Biol Anim. 2019. PMID: 31429037
-
Hypertonic stress induces rapid and widespread protein damage in C. elegans.Am J Physiol Cell Physiol. 2011 Sep;301(3):C566-76. doi: 10.1152/ajpcell.00030.2011. Epub 2011 May 25. Am J Physiol Cell Physiol. 2011. PMID: 21613604 Free PMC article.
References
-
- Schöls L., Bauer P., Schmidt T., Schulte T., Riess O. (2004) Lancet Neurol. 3, 291–304 - PubMed
-
- Cagnoli C., Mariotti C., Taroni F., Seri M., Brussino A., Michielotto C., Grisoli M., Di Bella D., Migone N., Gellera C., Di Donato S., Brusco A. (2006) Brain 129, 235–242 - PubMed
-
- Dueñas A. M., Goold R., Giunti P. (2006) Brain 129, 1357–1370 - PubMed
-
- Martin J. B. (1999) N. Engl. J. Med. 340, 1970–1980 - PubMed
-
- Everett C. M., Wood N. W. (2004) Brain 127, 2385–2405 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

