A flexible loop in yeast ribosomal protein L11 coordinates P-site tRNA binding
- PMID: 20705654
- PMCID: PMC3001080
- DOI: 10.1093/nar/gkq711
A flexible loop in yeast ribosomal protein L11 coordinates P-site tRNA binding
Abstract
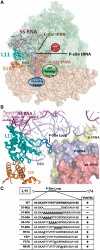
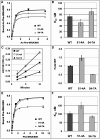
High-resolution structures reveal that yeast ribosomal protein L11 and its bacterial/archael homologs called L5 contain a highly conserved, basically charged internal loop that interacts with the peptidyl-transfer RNA (tRNA) T-loop. We call this the L11 'P-site loop'. Chemical protection of wild-type ribosome shows that that the P-site loop is inherently flexible, i.e. it is extended into the ribosomal P-site when this is unoccupied by tRNA, while it is retracted into the terminal loop of 25S rRNA Helix 84 when the P-site is occupied. To further analyze the function of this structure, a series of mutants within the P-site loop were created and analyzed. A mutant that favors interaction of the P-site loop with the terminal loop of Helix 84 promoted increased affinity for peptidyl-tRNA, while another that favors its extension into the ribosomal P-site had the opposite effect. The two mutants also had opposing effects on binding of aa-tRNA to the ribosomal A-site, and downstream functional effects were observed on translational fidelity, drug resistance/hypersensitivity, virus maintenance and overall cell growth. These analyses suggest that the L11 P-site loop normally helps to optimize ribosome function by monitoring the occupancy status of the ribosomal P-site.
Figures



Similar articles
-
Yeast ribosomal protein L10 helps coordinate tRNA movement through the large subunit.Nucleic Acids Res. 2008 Nov;36(19):6187-98. doi: 10.1093/nar/gkn643. Epub 2008 Sep 29. Nucleic Acids Res. 2008. PMID: 18824477 Free PMC article.
-
The central core region of yeast ribosomal protein L11 is important for subunit joining and translational fidelity.Mol Genet Genomics. 2011 Jun;285(6):505-16. doi: 10.1007/s00438-011-0623-2. Epub 2011 Apr 26. Mol Genet Genomics. 2011. PMID: 21519857 Free PMC article.
-
Ribosomal protein L5 helps anchor peptidyl-tRNA to the P-site in Saccharomyces cerevisiae.RNA. 2001 Aug;7(8):1084-96. doi: 10.1017/s1355838201001480. RNA. 2001. PMID: 11497428 Free PMC article.
-
The 30S ribosomal P site: a function of 16S rRNA.FEBS Lett. 2005 Feb 7;579(4):855-8. doi: 10.1016/j.febslet.2004.11.026. FEBS Lett. 2005. PMID: 15680962 Review.
-
The social life of ribosomal proteins.FEBS J. 2005 May;272(9):2098-108. doi: 10.1111/j.1742-4658.2005.04651.x. FEBS J. 2005. PMID: 15853795 Review.
Cited by
-
Structural snapshots of actively translating human ribosomes.Cell. 2015 May 7;161(4):845-57. doi: 10.1016/j.cell.2015.03.052. Cell. 2015. PMID: 25957688 Free PMC article.
-
Tracking fluctuation hotspots on the yeast ribosome through the elongation cycle.Nucleic Acids Res. 2017 May 5;45(8):4958-4971. doi: 10.1093/nar/gkx112. Nucleic Acids Res. 2017. PMID: 28334755 Free PMC article.
-
Evolution of ribosomal protein network architectures.Sci Rep. 2021 Jan 12;11(1):625. doi: 10.1038/s41598-020-80194-4. Sci Rep. 2021. PMID: 33436806 Free PMC article.
-
The eukaryote-specific N-terminal extension of ribosomal protein S31 contributes to the assembly and function of 40S ribosomal subunits.Nucleic Acids Res. 2016 Sep 19;44(16):7777-91. doi: 10.1093/nar/gkw641. Epub 2016 Jul 15. Nucleic Acids Res. 2016. PMID: 27422873 Free PMC article.
-
Eukaryotic rpL10 drives ribosomal rotation.Nucleic Acids Res. 2014 Feb;42(3):2049-63. doi: 10.1093/nar/gkt1107. Epub 2013 Nov 8. Nucleic Acids Res. 2014. PMID: 24214990 Free PMC article.
References
-
- Ban N, Nissen P, Hansen J, Moore PB, Steitz TA. The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science. 2000;289:905–920. - PubMed
-
- Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM, Cate JH. Structures of the bacterial ribosome at 3.5 A resolution. Science. 2005;310:827–834. - PubMed
-
- Spahn CM, Beckmann R, Eswar N, Penczek PA, Sali A, Blobel G, Frank J. Structure of the 80S ribosome from Saccharomyces cerevisiae–tRNA- ribosome and subunit-subunit interactions. Cell. 2001;107:373–386. - PubMed
-
- Yusupov MM, Yusupova GZ, Baucom A, Lieberman K, Earnest TN, Cate JH, Noller HF. Crystal structure of the ribosome at 5.5 Å resolution. Science. 2001;292:883–896. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

