Heterogeneous distribution of Candida albicans cell-surface antigens demonstrated with an Als1-specific monoclonal antibody
- PMID: 20705663
- PMCID: PMC3068703
- DOI: 10.1099/mic.0.043851-0
Heterogeneous distribution of Candida albicans cell-surface antigens demonstrated with an Als1-specific monoclonal antibody
Abstract
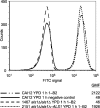
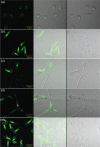
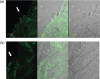
Despite an abundance of data describing expression of genes in the Candida albicans ALS (agglutinin-like sequence) gene family, little is known about the production of Als proteins on individual cells, their spatial localization or stability. Als proteins are most commonly discussed with respect to function in adhesion of C. albicans to host and abiotic surfaces. Development of a mAb specific for Als1, one of the eight large glycoproteins encoded by the ALS family, provided the opportunity to detect Als1 during growth of yeast and hyphae, both in vitro and in vivo, and to demonstrate the utility of the mAb in blocking C. albicans adhesion to host cells. Although most C. albicans yeast cells in a saturated culture are Als1-negative by indirect immunofluorescence, Als1 is detected on the surface of nearly all cells shortly after transfer into fresh growth medium. Als1 covers the yeast cell surface, with the exception of bud scars. Daughters of the inoculum cells, and sometimes granddaughters, also have detectable Als1, but Als1 is not detectable on cells from subsequent generations. On germ tubes and hyphae, most Als1 is localized proximal to the mother yeast. Once deposited on yeasts or hyphae, Als1 persists long after the culture has reached saturation. Growth stage-dependent production of Als1, coupled with its persistence on the cell surface, results in a heterogeneous population of cells within a C. albicans culture. Anti-Als1 immunolabelling patterns vary depending on the source of the C. albicans cells, with obvious differences between cells recovered from culture and those from a murine model of disseminated candidiasis. Results from this work highlight the temporal parallels for ALS1 expression and Als1 production in yeasts and germ tubes, the specialized spatial localization and persistence of Als1 on the C. albicans cell surface, and the differences in Als1 localization that occur in vitro and in vivo.
Figures






References
-
- Bi, E. (2001). Cytokinesis in budding yeast: the relationship between actomyosin ring function and septum formation. Cell Struct Funct 26, 529–537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

