A streptococcal effector protein that inhibits Porphyromonas gingivalis biofilm development
- PMID: 20705665
- PMCID: PMC7336479
- DOI: 10.1099/mic.0.042671-0
A streptococcal effector protein that inhibits Porphyromonas gingivalis biofilm development
Abstract
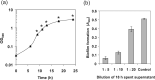
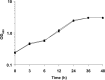
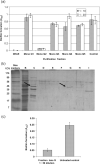
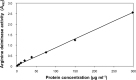
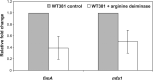
Dental plaque formation is a developmental process involving cooperation and competition within a diverse microbial community, approximately 70 % of which is composed of an array of streptococci during the early stages of supragingival plaque formation. In this study, 79 cell-free culture supernatants from a variety of oral streptococci were screened to identify extracellular compounds that inhibit biofilm formation by the oral anaerobe Porphyromonas gingivalis strain 381. The majority of the streptococcal supernatants (61 isolates) resulted in lysis of P. gingivalis cells, and some (17 isolates) had no effect on cell viability, growth or biofilm formation. One strain, however, produced a supernatant that abolished biofilm formation without affecting growth rate. Analysis of this activity led to the discovery that a 48 kDa protein was responsible for the inhibition. Protein sequence identification and enzyme activity assays identified the effector protein as an arginine deiminase. To identify the mechanism(s) by which this protein inhibits biofilm formation, we began by examining the expression levels of genes encoding fimbrial subunits; surface structures known to be involved in biofilm development. Quantitative RT-PCR analysis revealed that exposure of P. gingivalis cells to this protein for 1 h resulted in the downregulation of genes encoding proteins that are the major subunits of two distinct types of thin, single-stranded fimbriae (fimA and mfa1). Furthermore, this downregulation occurred in the absence of arginine deiminase enzymic activity. Hence, our data indicate that P. gingivalis can sense this extracellular protein, produced by an oral streptococcus (Streptococcus intermedius), and respond by downregulating expression of cell-surface appendages required for attachment and biofilm development.
Figures
References
-
- Amano A., Nakagawa I., Okahashi N., Hamada N. Variations of Porphyromonas gingivalis fimbriae in relation to microbial pathogenesis. J Periodontal Res. 2004;39:136–142. - PubMed
-
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Burne R. A., Marquis R. E. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol Lett. 2000;193:1–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

