G protein-coupled receptor kinase 2 and arrestin2 regulate arterial smooth muscle P2Y-purinoceptor signalling
- PMID: 20705669
- PMCID: PMC3002865
- DOI: 10.1093/cvr/cvq249
G protein-coupled receptor kinase 2 and arrestin2 regulate arterial smooth muscle P2Y-purinoceptor signalling
Abstract
Aims: prolonged P2Y-receptor signalling can cause vasoconstriction leading to hypertension, vascular smooth muscle hypertrophy, and hyperplasia. G protein-coupled receptor signalling is negatively regulated by G protein-coupled receptor kinases (GRKs) and arrestin proteins, preventing prolonged or inappropriate signalling. This study investigates whether GRKs and arrestins regulate uridine 5'-triphosphate (UTP)-stimulated contractile signalling in adult Wistar rat mesenteric arterial smooth muscle cells (MSMCs).
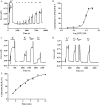
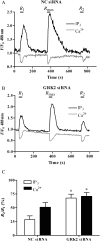
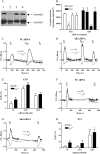
Methods and results: mesenteric arteries contracted in response to UTP challenge: When an EC(50) UTP concentration (30 µM, 5 min) was added 5 min before (R(1)) and after (R(2)) the addition of a maximal UTP concentration (R(max): 100 µM, 5 min), R(2) responses were decreased relative to R(1), indicating desensitization. UTP-induced P2Y-receptor desensitization of phospholipase C signalling was studied in isolated MSMCs transfected with an inositol 1,4,5-trisphosphate biosensor and/or loaded with Ca(2+)-sensitive dyes. A similar protocol (R(1)/R(2) = 10 µM; R(max) = 100 µM, applied for 30 s) revealed markedly reduced R(2) when compared with R(1) responses. MSMCs were transfected with dominant-negative GRKs or siRNAs targeting specific GRK/arrestins to probe their respective roles in P2Y-receptor desensitization. GRK2 inhibition, but not GRK3, GRK5, or GRK6, attenuated P2Y-receptor desensitization. siRNA-mediated knockdown of arrestin2 attenuated UTP-stimulated P2Y-receptor desensitization, whereas arrestin3 depletion did not. Specific siRNA knockdown of the P2Y(2)-receptor almost completely abolished UTP-stimulated IP(3)/Ca(2+) signalling, strongly suggesting that our study is specifically characterizing this purinoceptor subtype.
Conclusion: these new data highlight roles of GRK2 and arrestin2 as important regulators of UTP-stimulated P2Y(2)-receptor responsiveness in resistance arteries, emphasizing their potential importance in regulating vasoconstrictor signalling pathways implicated in vascular disease.
Figures



References
-
- Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4:1–20. doi:10.1007/s11302-007-9078-7. - DOI - PMC - PubMed
-
- Winter P, Dora KA. Spreading dilatation to luminal perfusion of ATP and UTP in rat isolated small mesenteric arteries. J Physiol. 2007;582:335–347. doi:10.1113/jphysiol.2007.135202. - DOI - PMC - PubMed
-
- von Kugelgen I, Haussinger D, Starke K. Evidence for a vasoconstriction-mediating receptor for UTP, distinct from the P2 purinoceptor, in rabbit ear artery. Naunyn Schmiedeberg's Arch Pharmacol. 1987;336:556–560. - PubMed
-
- Malmsjo M, Adner M, Harden TK, Pendergast W, Edvinsson L, Erlinge D. The stable pyrimidines UDPβS and UTPγS discriminate between the P2 receptors that mediate vascular contraction and relaxation of the rat mesenteric artery. Br J Pharmacol. 2000;131:51–56. doi:10.1038/sj.bjp.0703536. - DOI - PMC - PubMed
-
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, et al. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi:10.1124/pr.58.3.3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

