The different shades of mammalian pluripotent stem cells
- PMID: 20705693
- PMCID: PMC3039219
- DOI: 10.1093/humupd/dmq035
The different shades of mammalian pluripotent stem cells
Abstract
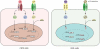
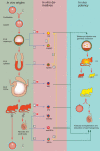
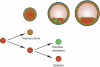
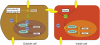
Background: Pluripotent stem cells have been derived from a variety of sources such as from the inner cell mass of preimplantation embryos, from primordial germ cells, from teratocarcinomas and from male germ cells. The recent development of induced pluripotent stem cells demonstrates that somatic cells can be reprogrammed to a pluripotent state in vitro.
Methods: This review summarizes our current understanding of the origins of mouse and human pluripotent cells. We pay specific attention to transcriptional and epigenetic regulation in pluripotent cells and germ cells. Furthermore, we discuss developmental aspects in the germline that seem to be of importance for the transition of germ cells towards pluripotency. This review is based on literature from the Pubmed database, using Boolean search statements with relevant keywords on the subject.
Results: There are distinct molecular mechanisms involved in the generation and maintenance of the various pluripotent cell types. Furthermore, there are important similarities and differences between the different categories of pluripotent cells in terms of phenotype and epigenetic modifications. Pluripotent cell lines from various origins differ in growth characteristics, developmental potential, transcriptional activity and epigenetic regulation. Upon derivation, pluripotent stem cells generally acquire new properties, but they often also retain a 'footprint' of their tissue of origin.
Conclusions: In order to further our knowledge of the mechanisms underlying self-renewal and pluripotency, a thorough comparison between different pluripotent stem cell types is required. This will progress the use of stem cells in basic biology, drug discovery and future clinical applications.
Figures

References
-
- Andrews PW. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol. 1984;103:285–293. doi:10.1016/0012-1606(84)90316-6. - DOI - PubMed
-
- Andrews PW. Human teratocarcinomas. Biochim Biophys Acta. 1988;948:17–36. - PubMed
-
- Andrews PW, Damjanov I, Simon D, Banting GS, Carlin C, Dracopoli NC, Fogh J. Pluripotent embryonal carcinoma clones derived from the human teratocarcinoma cell line Tera-2. Differentiation in vivo and in vitro. Lab Invest. 1984;50:147–162. - PubMed
-
- Arman E, Haffner-Krausz R, Chen Y, Heath JK, Lonai P. Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc Natl Acad Sci USA. 1998;95:5082–5087. doi:10.1073/pnas.95.9.5082. - DOI - PMC - PubMed

