Heme oxygenase-1 inhibits renal tubular macroautophagy in acute kidney injury
- PMID: 20705711
- PMCID: PMC3013546
- DOI: 10.1681/ASN.2010030238
Heme oxygenase-1 inhibits renal tubular macroautophagy in acute kidney injury
Abstract
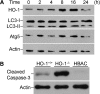
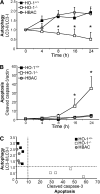
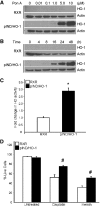
Autophagy is a tightly regulated, programmed mechanism to eliminate damaged organelles and proteins from a cell to maintain homeostasis. Cisplatin, a chemotherapeutic agent, accumulates in the proximal tubules of the kidney and causes dose-dependent nephrotoxicity, which may involve autophagy. In the kidney, cisplatin induces the protective antioxidant heme oxygenase-1 (HO-1). In this study, we examined the relationship between autophagy and HO-1 during cisplatin-mediated acute kidney injury (AKI). In wild-type primary proximal tubule cells (PTC), we observed a time-dependent increase in autophagy after cisplatin. In HO-1(-/-) PTC, however, we observed significantly higher levels of basal autophagy, impaired progression of autophagy, and increased apoptosis after cisplatin. Restoring HO-1 expression in these cells reversed the autophagic response and inhibited apoptosis after treatment with cisplatin. In vivo, although both wild-type and HO-1-deficient mice exhibited autophagosomes in the proximal tubules of the kidney in response to cisplatin, HO-1-deficient mice had significantly more autophagosomes, even in saline-treated animals. In addition, ecdysone-induced overexpression of HO-1 in cells led to a delay in autophagy progression, generated significantly lower levels of reactive oxygen species, and protected against cisplatin cytotoxicity. These findings demonstrate that HO-1 inhibits autophagy, suggesting that the heme oxygenase system may contain therapeutic targets for AKI.
Figures







Comment in
-
HO-1 in control of a self-eating kidney.J Am Soc Nephrol. 2010 Oct;21(10):1600-2. doi: 10.1681/ASN.2010080876. Epub 2010 Sep 9. J Am Soc Nephrol. 2010. PMID: 20829407 Free PMC article. No abstract available.
References
-
- Baliga R, Ueda N, Walker PD, Shah SV: Oxidant mechanisms in toxic acute renal failure. Am J Kidney Dis 29: 465–477, 1997 - PubMed
-
- Devarajan P: Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006 - PubMed
-
- Agarwal A, Balla J, Alam J, Croatt AJ, Nath KA: Induction of heme oxygenase in toxic renal injury: A protective role in cisplatin nephrotoxicity in the rat. Kidney Int 48: 1298–1307, 1995 - PubMed
-
- Nath KA: Heme oxygenase-1: A provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int 70: 432–443, 2006 - PubMed
-
- Agarwal A, Nick HS: Renal response to tissue injury: Lessons from heme oxygenase-1 gene ablation and expression. J Am Soc Nephrol 11: 965–973, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

