Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B
- PMID: 20705815
- PMCID: PMC3177562
- DOI: 10.1126/science.1189505
Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B
Abstract
A hallmark of mitosis is the appearance of high levels of histone phosphorylation, yet the roles of these modifications remain largely unknown. Here, we demonstrate that histone H3 phosphorylated at threonine 3 is directly recognized by an evolutionarily conserved binding pocket in the BIR domain of Survivin, which is a member of the chromosomal passenger complex (CPC). This binding mediates recruitment of the CPC to chromosomes and the resulting activation of its kinase subunit Aurora B. Consistently, modulation of the kinase activity of Haspin, which phosphorylates H3T3, leads to defects in the Aurora B-dependent processes of spindle assembly and inhibition of nuclear reformation. These findings establish a direct cellular role for mitotic histone H3T3 phosphorylation, which is read and translated by the CPC to ensure accurate cell division.
Figures

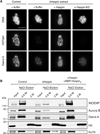
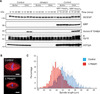
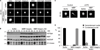
Comment in
-
Molecular biology. Surfing chromosomes (and Survivin).Science. 2010 Oct 8;330(6001):183-4. doi: 10.1126/science.1197261. Science. 2010. PMID: 20929762 No abstract available.
References
-
- Ubersax JA, Ferrell JE., Jr Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol. 2007;8:530. - PubMed
-
- Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798. - PubMed
-
- Sampath SC, et al. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell. 2004;118:187. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

