Dendritic discrimination of temporal input sequences in cortical neurons
- PMID: 20705816
- PMCID: PMC6354899
- DOI: 10.1126/science.1189664
Dendritic discrimination of temporal input sequences in cortical neurons
Abstract
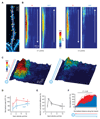
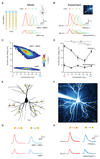
The detection and discrimination of temporal sequences is fundamental to brain function and underlies perception, cognition, and motor output. By applying patterned, two-photon glutamate uncaging, we found that single dendrites of cortical pyramidal neurons exhibit sensitivity to the sequence of synaptic activation. This sensitivity is encoded by both local dendritic calcium signals and somatic depolarization, leading to sequence-selective spike output. The mechanism involves dendritic impedance gradients and nonlinear synaptic N-methyl-D-aspartate receptor activation and is generalizable to dendrites in different neuronal types. This enables discrimination of patterns delivered to a single dendrite, as well as patterns distributed randomly across the dendritic tree. Pyramidal cell dendrites can thus act as processing compartments for the detection of synaptic sequences, thereby implementing a fundamental cortical computation.
Figures


Comment in
-
Neuroscience. Dendrites do it in sequences.Science. 2010 Sep 24;329(5999):1611-2. doi: 10.1126/science.1196743. Science. 2010. PMID: 20929837 No abstract available.
References
-
- Meister M, Lagnado L, Baylor DA. Science. 1995;270:1207. - PubMed
-
- deCharms RC, Merzenich MM. Nature. 1996;381:610. - PubMed
-
- Wehr M, Laurent G. Nature. 1996;384:162. - PubMed
-
- Johansson RS, Birznieks I. Nat Neurosci. 2004;7:170. - PubMed
-
- Thorpe S, Delorme A, Van Rullen R. Neural Netw. 2001;14:715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

