Fast live simultaneous multiwavelength four-dimensional optical microscopy
- PMID: 20705899
- PMCID: PMC2941331
- DOI: 10.1073/pnas.1004037107
Fast live simultaneous multiwavelength four-dimensional optical microscopy
Abstract
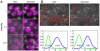
Live fluorescence microscopy has the unique capability to probe dynamic processes, linking molecular components and their localization with function. A key goal of microscopy is to increase spatial and temporal resolution while simultaneously permitting identification of multiple specific components. We demonstrate a new microscope platform, OMX, that enables subsecond, multicolor four-dimensional data acquisition and also provides access to subdiffraction structured illumination imaging. Using this platform to image chromosome movement during a complete yeast cell cycle at one 3D image stack per second reveals an unexpected degree of photosensitivity of fluorophore-containing cells. To avoid perturbation of cell division, excitation levels had to be attenuated between 100 and 10,000× below the level normally used for imaging. We show that an image denoising algorithm that exploits redundancy in the image sequence over space and time allows recovery of biological information from the low light level noisy images while maintaining full cell viability with no fading.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
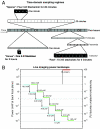

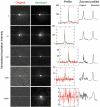

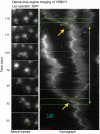

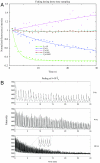
Comment in
-
Advanced hardware and software tools for fast multidimensional imaging of living cells.Proc Natl Acad Sci U S A. 2010 Sep 14;107(37):16005-6. doi: 10.1073/pnas.1010043107. Epub 2010 Aug 31. Proc Natl Acad Sci U S A. 2010. PMID: 20807743 Free PMC article. No abstract available.
-
Seeing more with less.Nat Methods. 2010 Oct;7(10):782. doi: 10.1038/nmeth1010-782. Nat Methods. 2010. PMID: 20936773 No abstract available.
References
-
- Pawley JB. Handbook of Biological Confocal Microscopy. 2nd Ed. New York: Plenum; 2006.
-
- Jamieson DJ. Oxidative stress responses of the yeast Saccharomyces cerevisiae. Yeast. 1998;14:1511–1527. - PubMed
-
- Straight AF, et al. GFP tagging of budding yeast chromosomes reveals that protein–protein interactions can mediate sister chromatid cohesion. Curr Biol. 1996;6:1599–1608. - PubMed
-
- Weinert TA, Hartwell LH. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science. 1988;241:317–322. - PubMed
-
- Boulanger J, Kervrann C, Bouthemy P. Space-time adaptation for patch-based image sequence restoration. IEEE T Pattern Anal. 2007;29:1096–1102. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

