Bifidobacterium bifidum reduces apoptosis in the intestinal epithelium in necrotizing enterocolitis
- PMID: 20705904
- PMCID: PMC2993641
- DOI: 10.1152/ajpgi.00131.2010
Bifidobacterium bifidum reduces apoptosis in the intestinal epithelium in necrotizing enterocolitis
Abstract
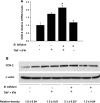
Necrotizing enterocolitis (NEC) is a devastating intestinal disease of neonates, and clinical studies suggest the beneficial effect of probiotics in NEC prevention. Recently, we have shown that administration of Bifidobacterium bifidum protects against NEC in a rat model. Intestinal apoptosis can be suppressed by activation of cyclooxygenase-2 (COX-2) and increased production of prostaglandin E(2) (PGE(2)). The present study investigates the effect of B. bifidum on intestinal apoptosis in the rat NEC model and in an intestinal epithelial cell line (IEC-6), as a mechanism of protection against mucosal injury. Premature rats were divided into the following three groups: dam fed, hand fed with formula (NEC), or hand fed with formula supplemented with B. bifidum (NEC + B. bifidum). Intestinal Toll-like receptor-2 (TLR-2), COX-2, PGE(2), and apoptotic regulators were measured. The effect of B. bifidum was verified in IEC-6 cells using a model of cytokine-induced apoptosis. Administration of B. bifidum increased expression of TLR-2, COX-2, and PGE(2) and significantly reduced apoptosis in the intestinal epithelium of both in vivo and in vitro models. The Bax-to-Bcl-w ratio was shifted toward cell survival, and the number of cleaved caspase-3 positive cells was markedly decreased in B. bifidum-treated rats. Experiments in IEC-6 cells showed anti-apoptotic effect of B. bifidum. Inhibition of COX-2 signaling blocked the protective effect of B. bifidum treatment in both in vivo and in vitro models. In conclusion, oral administration of B. bifidum activates TLR-2 in the intestinal epithelium. B. bifidum increases expression of COX-2, which leads to higher production of PGE(2) in the ileum and protects against intestinal apoptosis associated with NEC. This study indicates the ability of B. bifidum to downregulate apoptosis in the rat NEC model and in IEC-6 cells by a COX-2-dependent matter and suggests a molecular mechanism by which this probiotic reduces mucosal injury and preserves intestinal integrity.
Figures





References
-
- Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, Hammerman C. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr 147: 192–196, 2005 - PubMed
-
- Bos J, Smithee L, McClane B, Distefano RF, Uzal F, Songer JG, Mallonee S, Crutcher JM. Fatal necrotizing colitis following a foodborne outbreak of enterotoxigenic Clostridium perfringens type A infection. Clin Infect Dis 40: e78–e83, 2005 - PubMed
-
- Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 132: 1359–1374, 2007 - PubMed
-
- Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 127: 224–238, 2004 - PubMed
-
- Chang YJ, Wu MS, Lin JT, Sheu BS, Muta T, Inoue H, Chen CC. Induction of cyclooxygenase-2 overexpression in human gastric epithelial cells by Helicobacter pylori involves TLR2/TLR9 and c-Src-dependent nuclear factor-kappaB activation. Mol Pharmacol 66: 1465–1477, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

