Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells
- PMID: 20705905
- PMCID: PMC3774266
- DOI: 10.1152/ajpgi.00156.2010
Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells
Abstract
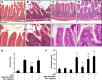
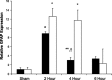
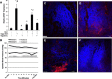
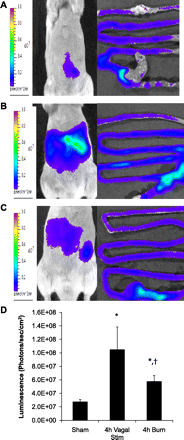
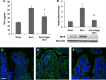
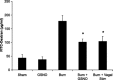
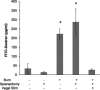
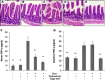
The enteric nervous system may have an important role in modulating gastrointestinal barrier response to disease through activation of enteric glia cells. In vitro studies have shown that enteric glia activation improves intestinal epithelial barrier function by altering the expression of tight junction proteins. We hypothesized that severe injury would increase expression of glial fibrillary acidic protein (GFAP), a marker of enteric glial activation. We also sought to define the effects of vagal nerve stimulation on enteric glia activation and intestinal barrier function using a model of systemic injury and local gut mucosal involvement. Mice with 30% total body surface area steam burn were used as model of severe injury. Vagal nerve stimulation was performed to assess the role of parasympathetic signaling on enteric glia activation. In vivo intestinal permeability was measured to assess barrier function. Intestine was collected to investigate changes in histology; GFAP expression was assessed by quantitative PCR, by confocal microscopy, and in GFAP-luciferase transgenic mice. Stimulation of the vagus nerve prevented injury-induced intestinal barrier injury. Intestinal GFAP expression increased at early time points following burn and returned to baseline by 24 h after injury. Vagal nerve stimulation prior to injury increased GFAP expression to a greater degree than burn alone. Gastrointestinal bioluminescence was imaged in GFAP-luciferase transgenic animals following either severe burn or vagal stimulation and confirmed the increased expression of intestinal GFAP. Injection of S-nitrosoglutathione, a signaling molecule released by activated enteric glia cells, following burn exerts protective effects similar to vagal nerve stimulation. Intestinal expression of GFAP increases following severe burn injury. Stimulation of the vagus nerve increases enteric glia activation, which is associated with improved intestinal barrier function. The vagus nerve may mediate the signaling that occurs from the central nervous system to the enteric nervous system following gastrointestinal injury.
Figures

Similar articles
-
Efferent vagal nerve stimulation attenuates gut barrier injury after burn: modulation of intestinal occludin expression.J Trauma. 2010 Jun;68(6):1349-54; discussion 1354-6. doi: 10.1097/TA.0b013e3181dccea0. J Trauma. 2010. PMID: 20539179 Free PMC article.
-
Stimulating the central nervous system to prevent intestinal dysfunction after traumatic brain injury.J Trauma. 2010 May;68(5):1059-64. doi: 10.1097/TA.0b013e3181d87373. J Trauma. 2010. PMID: 20453760 Free PMC article.
-
Enteric Glia Regulate Gastrointestinal Motility but Are Not Required for Maintenance of the Epithelium in Mice.Gastroenterology. 2017 Oct;153(4):1068-1081.e7. doi: 10.1053/j.gastro.2017.07.002. Epub 2017 Jul 13. Gastroenterology. 2017. PMID: 28711628 Free PMC article.
-
Anti-inflammatory effects of vagal nerve stimulation with a special attention to intestinal barrier dysfunction.Neurogastroenterol Motil. 2022 Oct;34(10):e14456. doi: 10.1111/nmo.14456. Epub 2022 Sep 12. Neurogastroenterol Motil. 2022. PMID: 36097404 Free PMC article. Review.
-
Enteric glia.Neurogastroenterol Motil. 2004 Apr;16 Suppl 1:44-9. doi: 10.1111/j.1743-3150.2004.00474.x. Neurogastroenterol Motil. 2004. PMID: 15066004 Review.
Cited by
-
Enteric glial biology, intercellular signalling and roles in gastrointestinal disease.Nat Rev Gastroenterol Hepatol. 2021 Aug;18(8):571-587. doi: 10.1038/s41575-021-00423-7. Epub 2021 Mar 17. Nat Rev Gastroenterol Hepatol. 2021. PMID: 33731961 Free PMC article. Review.
-
Effects of Glutathione on Growth, Intestinal Antioxidant Capacity, Histology, Gene Expression, and Microbiota of Juvenile Triploid Oncorhynchus mykiss.Front Physiol. 2021 Nov 29;12:784852. doi: 10.3389/fphys.2021.784852. eCollection 2021. Front Physiol. 2021. PMID: 34925074 Free PMC article.
-
Regulation of the Autonomic Nervous System on Intestine.Front Physiol. 2021 Jul 14;12:700129. doi: 10.3389/fphys.2021.700129. eCollection 2021. Front Physiol. 2021. PMID: 34335306 Free PMC article. Review.
-
Enteric glia cells attenuate cytomix-induced intestinal epithelial barrier breakdown.PLoS One. 2013 Jul 1;8(7):e69042. doi: 10.1371/journal.pone.0069042. Print 2013. PLoS One. 2013. PMID: 23840906 Free PMC article.
-
Recognizing the role of the vagus nerve in depression from microbiota-gut brain axis.Front Neurol. 2022 Nov 10;13:1015175. doi: 10.3389/fneur.2022.1015175. eCollection 2022. Front Neurol. 2022. PMID: 36438957 Free PMC article. Review.
References
-
- Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev 87: 545–564, 2007 - PubMed
-
- Bradley JS, Jr, Parr EJ, Sharkey KA. Effects of inflammation on cell proliferation in the myenteric plexus of the guinea-pig ileum. Cell Tissue Res 289: 455–461, 1997 - PubMed
-
- Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell 93: 189–201, 1998 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

