Enhanced fibroblast-myocyte interactions in response to cardiac injury
- PMID: 20705922
- PMCID: PMC2993566
- DOI: 10.1161/CIRCRESAHA.110.227421
Enhanced fibroblast-myocyte interactions in response to cardiac injury
Abstract
Rationale: A critical event in the development of cardiac fibrosis is the transformation of fibroblasts into myofibroblasts. The electrophysiological consequences of this phenotypic switch remain largely unknown.
Objective: Determine whether fibroblast activation following cardiac injury results in a distinct electrophysiological phenotype that enhances fibroblast-myocyte interactions.
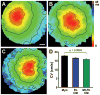
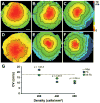
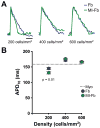
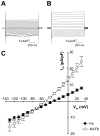
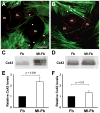
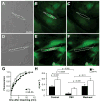
Methods and results: Neonatal rat myocyte monolayers were treated with media (CM) conditioned by fibroblasts isolated from normal (Fb) and infarcted (MI-Fb) hearts. Fb and MI-Fb were also plated on top of myocyte monolayers at 3 densities. Cultures were optically mapped after CM treatment or fibroblast plating to obtain conduction velocity and action potential duration (APD(70)). Intercellular communication and connexin43 expression levels were assessed. Membrane properties of Fb and MI-Fb were evaluated using patch clamp techniques. MI-Fb CM treatment decreased conduction velocity (11.1%) compared to untreated myocyte cultures. APD(70) was reduced by MI-Fb CM treatment compared to homocellular myocyte culture (9.4%) and Fb CM treatment (6.4%). In heterocellular cultures, MI-Fb conduction velocities were different from Fb at all densities (+29.8%, -23.0%, and -16.7% at 200, 400, and 600 cells/mm(2), respectively). APD(70) was reduced (9.6%) in MI-Fb compared to Fb cultures at 200 cells/mm(2). MI-Fb had more hyperpolarized resting membrane potentials and increased outward current densities. Connexin43 was elevated (134%) in MI-Fb compared to Fb. Intercellular coupling evaluated with gap fluorescence recovery after photobleaching was higher between myocytes and MI-Fb compared to Fb.
Conclusions: These data demonstrate cardiac injury results in significant electrophysiological changes that enhance fibroblast-myocyte interactions and could contribute to the greater incidence of arrhythmias observed in fibrotic hearts.
Figures
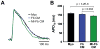
References
-
- Sappino AP, Schurch W, Gabbiani G. Differentiation repertoire of fibroblastic cells: Expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest. 1990;63:144–161. - PubMed
-
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277:C1–9. - PubMed
-
- Petrov VV, Fagard RH, Lijnen PJ. Stimulation of collagen production by transforming growth factor-beta1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension. 2002;39:258–263. - PubMed
-
- Wang J, Chen H, Seth A, McCulloch CA. Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2003;285:H1871–1881. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

