Ketogenic essential amino acids modulate lipid synthetic pathways and prevent hepatic steatosis in mice
- PMID: 20706589
- PMCID: PMC2919399
- DOI: 10.1371/journal.pone.0012057
Ketogenic essential amino acids modulate lipid synthetic pathways and prevent hepatic steatosis in mice
Abstract
Background: Although dietary ketogenic essential amino acid (KAA) content modifies accumulation of hepatic lipids, the molecular interactions between KAAs and lipid metabolism are yet to be fully elucidated.
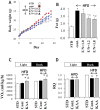
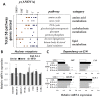
Methodology/principal findings: We designed a diet with a high ratio (E/N) of essential amino acids (EAAs) to non-EAAs by partially replacing dietary protein with 5 major free KAAs (Leu, Ile, Val, Lys and Thr) without altering carbohydrate and fat content. This high-KAA diet was assessed for its preventive effects on diet-induced hepatic steatosis and whole-animal insulin resistance. C57B6 mice were fed with a high-fat diet, and hyperinsulinemic ob/ob mice were fed with a high-fat or high-sucrose diet. The high-KAA diet improved hepatic steatosis with decreased de novo lipogenesis (DNL) fluxes as well as reduced expressions of lipogenic genes. In C57B6 mice, the high-KAA diet lowered postprandial insulin secretion and improved glucose tolerance, in association with restored expression of muscle insulin signaling proteins repressed by the high-fat diet. Lipotoxic metabolites and their synthetic fluxes were also evaluated with reference to insulin resistance. The high-KAA diet lowered muscle and liver ceramides, both by reducing dietary lipid incorporation into muscular ceramides and preventing incorporation of DNL-derived fatty acids into hepatic ceramides.
Conclusion: Our results indicate that dietary KAA intake improves hepatic steatosis and insulin resistance by modulating lipid synthetic pathways.
Conflict of interest statement
Figures






References
-
- Tilg H, Kaser A. Treatment strategies in nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:148–155. - PubMed
-
- Sanyal AJ. Mechanisms of Disease: pathogenesis of nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:46–53. - PubMed
-
- Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. - PubMed
-
- Chibalin AV, Leng Y, Vieira E, Krook A, Bjornholm M, et al. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell. 2008;132:375–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

