Biochemistry, molecular biology, and pharmacology of fatty acid synthase, an emerging therapeutic target and diagnosis/prognosis marker
- PMID: 20706604
- PMCID: PMC2919769
Biochemistry, molecular biology, and pharmacology of fatty acid synthase, an emerging therapeutic target and diagnosis/prognosis marker
Abstract
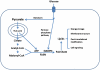
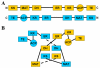
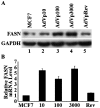
Human fatty acid synthase (FASN) is a 270-kDa cytosolic dimeric enzyme that is responsible for palmitate synthesis. FASN is slowly emerging and rediscovered as a marker for diagnosis and prognosis of human cancers. Recent studies showed that FASN is an oncogene and inhibition of FASN effectively and selectively kill cancer cells. With recent publications of the FASN crystal structure and the new development of FASN inhibitors, targeting FASN opens a new window of opportunity for metabolically combating cancers. In this article, we will review critically the recent progresses in understanding the structure, function, and the role of FASN in cancers and pharmacologically targeting FASN for human cancer treatment.
Keywords: Fatty acid synthase; cancer; diagnosis; drug resistance; inhibitors; prognosis.
Figures
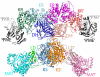

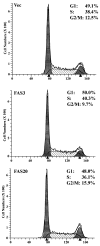
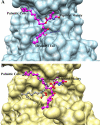
References
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. - PubMed
-
- Shaw RJ., Glucose metabolism and cancer. Curr Opin Cell Biol. 2006;18:598–608. - PubMed
-
- Bui T, Thompson CB. Cancer's sweet tooth. Cancer Cell. 2006;9:419–420. - PubMed
-
- Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16:202–208. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
