Dual effect of neutrophils on pIgR/secretory component in human bronchial epithelial cells: role of TGF-beta
- PMID: 20706611
- PMCID: PMC2914448
- DOI: 10.1155/2010/428618
Dual effect of neutrophils on pIgR/secretory component in human bronchial epithelial cells: role of TGF-beta
Abstract
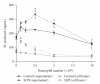
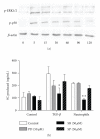
Neutrophils have a dual affect on epithelial pIgR/SC, the critical receptor for transcellular routing of mucosal IgA, but mechanisms of pIgR/SC upregulation remain elusive. Requirements of cytokine, redox, and signalling pathways for pIgR/SC production were assessed in human bronchial epithelial (Calu-3) cells cocultured with increasing numbers of blood neutrophils. Increased SC production was observed after incubation for 48 hrs with intermediate neutrophil numbers (1.25 to 2.5 x 10(6)), was favoured by the elastase inhibitor SLPI, and correlated with increased TGF-beta production. Exogenous TGF-beta stimulated SC production with a maximal effect at 48 hrs and both TGF-beta- and neutrophil-driven SC upregulation were dependent on redox balance and p38 MAP-kinase activation. This paper shows that activated neutrophils could upregulate epithelial pIgR/SC production through TGF-beta-mediated activation of a redox-sensitive and p38 MAPK-dependent pathway. An imbalance between the two neutrophil-driven opposite mechanisms (SC upregulation and SC degradation) could lead to downregulation of pIgR/SC, as observed in severe COPD.
Figures

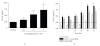


References
-
- Pilette C, Durham SR, Vaerman JP, Sibille Y. Mucosal immunity in asthma and chronic obstructive pulmonary disease: a role for immunoglobulin A? Proceedings of the American Thoracic Society. 2004;1(2):125–135. - PubMed
-
- Brandtzaeg P. Role of secretory antibodies in the defence against infections. International Journal of Medical Microbiology. 2003;293(1):3–15. - PubMed
-
- Kaetzel CS. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunological Reviews. 2005;206:83–99. - PubMed
-
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370(9589):765–773. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

