A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults
- PMID: 20706626
- PMCID: PMC2919376
- DOI: 10.1371/journal.pntd.0000785
A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults
Erratum in
- PLoS Negl Trop Dis. 2012 Jun;6(6). doi:10.1371/annotation/8683caec-b309-46d7-bc47-dc9cc27108e4
- PLoS Negl Trop Dis. 2012 Jun;6(6). doi:10.1371/annotation/c5c14905-8792-4d2e-8179-f8c70064e773
- PLoS Negl Trop Dis. 2012 Jun;6(6). doi:10.1371/annotation/e00ee8fb-4ab9-46db-be8e-3696bb362db4
Abstract
Background: There is currently no licensed antiviral drug for treatment of dengue. Chloroquine (CQ) inhibits the replication of dengue virus (DENV) in vitro.
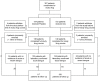
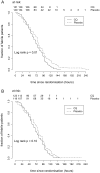
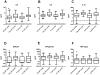
Methods and findings: A double-blind, randomized, placebo-controlled trial of CQ in 307 adults hospitalized for suspected DENV infection was conducted at the Hospital for Tropical Diseases (Ho Chi Minh City, Vietnam) between May 2007 and July 2008. Patients with illness histories of 72 hours or less were randomized to a 3-day course of CQ (n = 153) or placebo (n = 154). Laboratory-confirmation of DENV infection was made in 257 (84%) patients. The primary endpoints were time to resolution of DENV viraemia and time to resolution of DENV NS1 antigenaemia. In patients treated with CQ there was a trend toward a longer duration of DENV viraemia (hazard ratio (HR) = 0.80, 95% CI 0.62-1.05), but we did not find any difference for the time to resolution of NS1 antigenaemia (HR = 1.07, 95% CI 0.76-1.51). Interestingly, CQ was associated with a significant reduction in fever clearance time in the intention-to-treat population (HR = 1.37, 95% CI 1.08-1.74) but not in the per-protocol population. There was also a trend towards a lower incidence of dengue hemorrhagic fever (odds ratio = 0.60, PP 95% CI 0.34-1.04) in patients treated with CQ. Differences in levels of T cell activation or pro- or anti-inflammatory plasma cytokine concentrations between CQ- and placebo-treated patients did not explain the trend towards less dengue hemorrhagic fever in the CQ arm. CQ was associated with significantly more adverse events, primarily vomiting.
Conclusions: CQ does not reduce the durations of viraemia and NS1 antigenaemia in dengue patients. Further trials, with appropriate endpoints, would be required to determine if CQ treatment has any clinical benefit in dengue.
Trial registration: Current Controlled Trials number ISRCTN38002730.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- WHO. Dengue: guidelines for diagnosis, treatment, prevention and control - New edition.: 2009. World Health Organization, Geneva. - PubMed
-
- Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9. - PubMed
-
- Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, et al. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J Clin Microbiol. 2002;40:376–381. - PMC - PubMed
-
- Chuansumrit A, Chaiyaratana W, Pongthanapisith V, Tangnararatchakit K, Lertwongrath S, et al. The use of dengue nonstructural protein 1 antigen for the early diagnosis during the febrile stage in patients with dengue infection. Pediatr Infect Dis J. 2008;27:43–48. - PubMed
-
- Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, et al. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis. 2002;186:1165–1168. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

