Immunization with cocktail of HIV-derived peptides in montanide ISA-51 is immunogenic, but causes sterile abscesses and unacceptable reactogenicity
- PMID: 20706632
- PMCID: PMC2919382
- DOI: 10.1371/journal.pone.0011995
Immunization with cocktail of HIV-derived peptides in montanide ISA-51 is immunogenic, but causes sterile abscesses and unacceptable reactogenicity
Abstract
Background: A peptide vaccine was produced containing B and T cell epitopes from the V3 and C4 Envelope domains of 4 subtype B HIV-1 isolates (MN, RF, CanO, & Ev91). The peptide mixture was formulated as an emulsion in incomplete Freund's adjuvant (IFA).
Methods: Low-risk, healthy adult subjects were enrolled in a randomized, placebo-controlled dose-escalation study, and selected using criteria specifying that 50% in each study group would be HLA-B7+. Immunizations were scheduled at 0, 1, and 6 months using a total peptide dose of 1 or 4 mg. Adaptive immune responses in16 vaccine recipients and two placebo recipients after the 2nd immunization were evaluated using neutralization assays of sera, as well as ELISpot and ICS assays of cryopreserved PBMCs to assess CD4 and CD8 T-cell responses. In addition, (51)Cr release assays were performed on fresh PBMCs following 14-day stimulation with individual vaccine peptide antigens.
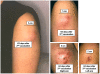
Results: 24 subjects were enrolled; 18 completed 2 injections. The study was prematurely terminated because 4 vaccinees developed prolonged pain and sterile abscess formation at the injection site-2 after dose 1, and 2 after dose 2. Two other subjects experienced severe systemic reactions consisting of headache, chills, nausea, and myalgia. Both reactions occurred after the second 4 mg dose. The immunogenicity assessments showed that 6/8 vaccinees at each dose level had detectable MN-specific neutralizing (NT) activity, and 2/7 HLA-B7+ vaccinees had classical CD8 CTL activity detected. However, using both ELISpot and ICS, 8/16 vaccinees (5/7 HLA-B7+) and 0/2 controls had detectable vaccine-specific CD8 T-cell responses. Subjects with moderate or severe systemic or local reactions tended to have more frequent T cell responses and higher antibody responses than those with mild or no reactions.
Conclusions: The severity of local responses related to the formulation of these four peptides in IFA is clinically unacceptable for continued development. Both HIV-specific antibody and T cell responses were induced and the magnitude of response correlated with the severity of local and systemic reactions. If potent adjuvants are necessary for subunit vaccines to induce broad and durable immune responses, careful, incremental clinical evaluation is warranted to minimize the risk of adverse events.
Trial registration: ClinicalTrials.gov NCT00000886.
Conflict of interest statement
Figures
References
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, et al. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N Engl J Med 2009 - PubMed
-
- Hart MK, Palker TJ, Matthews TJ, Langlois AJ, Lerche NW, et al. Synthetic peptides containing T and B cell epitopes from human immunodeficiency virus envelope gp120 induce anti-HIV proliferative responses and high titers of neutralizing antibodies in rhesus monkeys. J Immunol. 1990;145:2677–2685. - PubMed
-
- Lambert JS, Keefer M, Mulligan MJ, Schwartz D, Mestecky J, et al. A Phase I safety and immunogenicity trial of UBI microparticulate monovalent HIV-1 MN oral peptide immunogen with parenteral boost in HIV-1 seronegative human subjects. Vaccine. 2001;19:3033–3042. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

